Related Research Articles

A flare, also sometimes called a fusée, fusee, or bengala in some Latin-speaking countries, is a type of pyrotechnic that produces a bright light or intense heat without an explosion. Flares are used for distress signaling, illumination, or defensive countermeasures in civilian and military applications. Flares may be ground pyrotechnics, projectile pyrotechnics, or parachute-suspended to provide maximum illumination time over a large area. Projectile pyrotechnics may be dropped from aircraft, fired from rocket or artillery, or deployed by flare guns or handheld percussive tubes.

Thermite is a pyrotechnic composition of metal powder and metal oxide. When ignited by heat or chemical reaction, thermite undergoes an exothermic reduction-oxidation (redox) reaction. Most varieties are not explosive, but can create brief bursts of heat and high temperature in a small area. Its form of action is similar to that of other fuel-oxidizer mixtures, such as black powder.

Potassium chlorate is a compound containing potassium, chlorine and oxygen, with the molecular formula KClO3. In its pure form, it is a white crystalline substance. After sodium chlorate, it is the second most common chlorate in industrial use. It is a strong oxidizing agent and its most important application is in safety matches. In other applications it is mostly obsolete and has been replaced by safer alternatives in recent decades. It has been used

Tracer ammunition (tracers) are bullets or cannon-caliber projectiles that are built with a small pyrotechnic charge in their base. When fired, the pyrotechnic composition is ignited by the burning powder and burns very brightly, making the projectile trajectory visible to the naked eye during daylight, and very bright during nighttime firing. This allows the shooter to visually trace the flight path of the projectile and thus make necessary ballistic corrections, without having to confirm projectile impacts and without even using the sights of the weapon. Tracer fire can also be used as a marking tool to signal other shooters to concentrate their fire on a particular target during battle.

A sparkler is a type of hand-held firework that burns slowly while emitting bright, intense colored flames, sparks, and other effects.

In an explosive, pyrotechnic device, or military munition, a fuse is the part of the device that initiates function. In common usage, the word fuse is used indiscriminately. However, when being specific, the term fuse describes a simple pyrotechnic initiating device, like the cord on a firecracker whereas the term fuze is used when referring to a more sophisticated ignition device incorporating mechanical and/or electronic components, such as a proximity fuze for an M107 artillery shell, magnetic or acoustic fuze on a sea mine, spring-loaded grenade fuze, pencil detonator, or anti-handling device.

The fire triangle or combustion triangle is a simple model for understanding the necessary ingredients for most fires.

Flash powder is a pyrotechnic composition, a mixture of oxidizer and metallic fuel, which burns quickly and if confined produces a loud noise. It is widely used in theatrical pyrotechnics and fireworks and was once used for flashes in photography.
A chemical oxygen generator is a device that releases oxygen via a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate; ozonides are a promising group of oxygen sources. The generators are usually ignited by a firing pin, and the chemical reaction is usually exothermic, making the generator a potential fire hazard. Potassium superoxide was used as an oxygen source on early crewed missions of the Soviet space program, in submarines for use in emergency situations, for firefighters, and for mine rescue.
Nano-thermite or super-thermite is a metastable intermolecular composite (MIC) characterized by a particle size of its main constituents, a metal and a metal oxide, under 100 nanometers. This allows for high and customizable reaction rates. Nano-thermites contain an oxidizer and a reducing agent, which are intimately mixed on the nanometer scale. MICs, including nano-thermitic materials, are a type of reactive materials investigated for military use, as well as for general applications involving propellants, explosives, and pyrotechnics.

Colored fire is a common pyrotechnic effect used in stage productions, fireworks and by fire performers the world over. Generally, the color of a flame may be red, orange, blue, yellow, or white, and is dominated by blackbody radiation from soot and steam. When additional chemicals are added to the fuel burning, their atomic emission spectra can affect the frequencies of visible light radiation emitted - in other words, the flame appears in a different color dependent upon the chemical additives. Flame coloring is also a good way to demonstrate how fire changes when subjected to heat and how they also change the matter around them.

A pyrotechnic fastener is a fastener, usually a nut or bolt, that incorporates a pyrotechnic charge that can be initiated remotely. One or more explosive charges embedded within the bolt are typically activated by an electric current, and the charge breaks the bolt into two or more pieces. The bolt is typically scored around its circumference at the point(s) where the severance should occur. Such bolts are often used in space applications to ensure separation between rocket stages, because they are lighter and much more reliable than mechanical latches.

Molten-salt batteries are a class of battery that uses molten salts as an electrolyte and offers both a high energy density and a high power density. Traditional non-rechargeable thermal batteries can be stored in their solid state at room temperature for long periods of time before being activated by heating. Rechargeable liquid-metal batteries are used for industrial power backup, special electric vehicles and for grid energy storage, to balance out intermittent renewable power sources such as solar panels and wind turbines.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
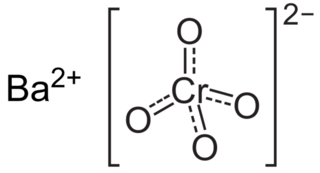
Barium chromate, named barium tetraoxochromate(VI) by the IUPAC, is a yellow sand like powder with the formula BaCrO4. It is a known oxidizing agent and produces a green flame when heated, a result of the barium ions.

A flare or decoy flare is an aerial infrared countermeasure used by a plane or helicopter to counter an infrared homing ("heat-seeking") surface-to-air missile or air-to-air missile. Flares are commonly composed of a pyrotechnic composition based on magnesium or another hot-burning metal, with burning temperature equal to or hotter than engine exhaust. The aim is to make the infrared-guided missile seek out the heat signature from the flare rather than the aircraft's engines.
A pyrotechnic initiator is a device containing a pyrotechnic composition used primarily to ignite other, more difficult-to-ignite materials, such as thermites, gas generators, and solid-fuel rockets. The name is often used also for the compositions themselves.
A smoke composition is a pyrotechnic composition designed primarily to generate smoke. Smoke compositions are used as obscurants or for generation of signaling smokes. Some are used as a payload of smoke bombs and smoke grenades.
Delay composition, also called delay charge or delay train, is a pyrotechnic composition, a sort of pyrotechnic initiator, a mixture of oxidizer and fuel that burns in a slow, constant rate that should not be significantly dependent on temperature and pressure. Delay compositions are used to introduce a delay into the firing train, e.g. to properly sequence firing of fireworks, to delay firing of ejection charges in e.g. model rockets, or to introduce a few seconds of time between triggering a hand grenade and its explosion. Typical delay times range between several milliseconds and several seconds.
Reactive multi-layer foils are a class of reactive materials, sometimes referred to as a pyrotechnic initiator of two mutually reactive metals, sputtered to form thin layers that create a laminated foil. On initiation by a heat pulse, delivered by a bridge wire, a laser pulse, an electric spark, a flame, or by other means, the metals undergo self-sustaining exothermic reaction, producing an intermetallic compound. The reaction occurs in solid and liquid phase only, without releasing any gas.
References
- ↑ Reed, J.W.; Walters, R.R.; Guidotti, R.A.; Jacobson, A.K. "Burn-rate studies with iron/potassium perchlorate heat pellets" IEEE 35th InternationalPower Sources Symposium, 1992., 22-25 Jun 1992 Page(s):211 - 214 doi : 10.1109/IPSS.1992.282041
- ↑ A High Temperature Molten Salt Thermal Electrochemical Cell. Oai.dtic.mil (1990-02-12). Retrieved on 2010-02-08.
- ↑ Heat transfer initiator - US20020035945. Patents.com. Retrieved on 2010-02-08.
- 1 2 SureChem. SureChem. Retrieved on 2010-02-08.
- ↑ (WO/2006/046245) HEAT SOURCES FOR THERMAL BATTERIES. Wipo.int. Retrieved on 2010-02-08.