
Charcot–Marie–Tooth disease (CMT) is a hereditary motor and sensory neuropathy of the peripheral nervous system characterized by progressive loss of muscle tissue and touch sensation across various parts of the body. This disease is the most commonly inherited neurological disorder, affecting about one in 2,500 people. It is named after those who classically described it: the Frenchman Jean-Martin Charcot (1825–1893), his pupil Pierre Marie (1853–1940), and the Briton Howard Henry Tooth (1856–1925).
Diabetic neuropathy is various types of nerve damage associated with diabetes mellitus. Symptoms depend on the site of nerve damage and can include motor changes such as weakness; sensory symptoms such as numbness, tingling, or pain; or autonomic changes such as urinary symptoms. These changes are thought to result from a microvascular injury involving small blood vessels that supply nerves. Relatively common conditions which may be associated with diabetic neuropathy include distal symmetric polyneuropathy; third, fourth, or sixth cranial nerve palsy; mononeuropathy; mononeuropathy multiplex; diabetic amyotrophy; and autonomic neuropathy.
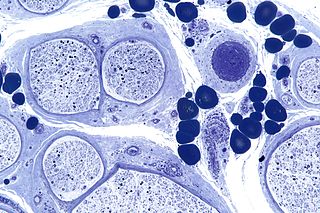
Peripheral neuropathy, often shortened to neuropathy, is a general term describing damage or disease affecting the nerves. Damage to nerves may impair sensation, movement, gland function, and/or organ function depending on which nerves are affected. Neuropathy affecting motor, sensory, or autonomic nerves result in different symptoms. More than one type of nerve may be affected simultaneously. Peripheral neuropathy may be acute or chronic, and may be reversible or permanent.

Polyneuropathy is damage or disease affecting peripheral nerves in roughly the same areas on both sides of the body, featuring weakness, numbness, and burning pain. It usually begins in the hands and feet and may progress to the arms and legs and sometimes to other parts of the body where it may affect the autonomic nervous system. It may be acute or chronic. A number of different disorders may cause polyneuropathy, including diabetes and some types of Guillain–Barré syndrome.

Hyperalgesia is an abnormally increased sensitivity to pain, which may be caused by damage to nociceptors or peripheral nerves and can cause hypersensitivity to stimulus. Prostaglandins E and F are largely responsible for sensitizing the nociceptors. Temporary increased sensitivity to pain also occurs as part of sickness behavior, the evolved response to infection.
Neuropathic pain is pain caused by a lesion or disease of the somatosensory nervous system. Neuropathic pain may be associated with abnormal sensations called dysesthesia or pain from normally non-painful stimuli (allodynia). It may have continuous and/or episodic (paroxysmal) components. The latter resemble stabbings or electric shocks. Common qualities include burning or coldness, "pins and needles" sensations, numbness and itching.
Neuralgia is pain in the distribution of a nerve or nerves, as in intercostal neuralgia, trigeminal neuralgia, and glossopharyngeal neuralgia.
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired autoimmune disease of the peripheral nervous system characterized by progressive weakness and impaired sensory function in the legs and arms. The disorder is sometimes called chronic relapsing polyneuropathy (CRP) or chronic inflammatory demyelinating polyradiculoneuropathy. CIDP is closely related to Guillain–Barré syndrome and it is considered the chronic counterpart of that acute disease. Its symptoms are also similar to progressive inflammatory neuropathy. It is one of several types of neuropathy.
Small fiber peripheral neuropathy is a type of peripheral neuropathy that occurs from damage to the small unmyelinated and myelinated peripheral nerve fibers. These fibers, categorized as C fibers and small Aδ fibers, are present in skin, peripheral nerves, and organs. The role of these nerves is to innervate some skin sensations and help control autonomic function. It is estimated that 15–20 million people in the United States have some form of peripheral neuropathy.

Group C nerve fibers are one of three classes of nerve fiber in the central nervous system (CNS) and peripheral nervous system (PNS). The C group fibers are unmyelinated and have a small diameter and low conduction velocity, whereas Groups A and B are myelinated. Group C fibers include postganglionic fibers in the autonomic nervous system (ANS), and nerve fibers at the dorsal roots. These fibers carry sensory information.
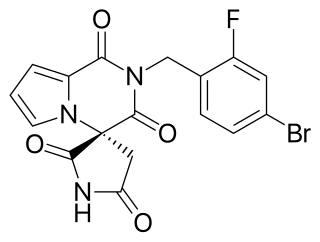
Ranirestat is an aldose reductase inhibitor being developed for the treatment of diabetic neuropathy by Dainippon Sumitomo Pharma and PharmaKyorin. It has been granted orphan drug status. The drug is to be used orally.

Nav1.8 is a sodium ion channel subtype that in humans is encoded by the SCN10A gene.
Anterior interosseous syndrome is a medical condition in which damage to the anterior interosseous nerve (AIN), a distal motor and sensory branch of the median nerve, classically with severe weakness of the pincer movement of the thumb and index finger, and can cause transient pain in the wrist.

Nerve compression syndrome, or compression neuropathy, or nerve entrapment syndrome, is a medical condition caused by chronic, direct pressure on a peripheral nerve. It is known colloquially as a trapped nerve, though this may also refer to nerve root compression. Its symptoms include pain, tingling, numbness and muscle weakness. The symptoms affect just one particular part of the body, depending on which nerve is affected. The diagnosis is largely clinical and can be confirmed with diagnostic nerve blocks. Occasionally imaging and electrophysiology studies aid in the diagnosis. Timely diagnosis is important as untreated chronic nerve compression may cause permanent damage. A surgical nerve decompression can relieve pressure on the nerve but cannot always reverse the physiological changes that occurred before treatment. Nerve injury by a single episode of physical trauma is in one sense an acute compression neuropathy but is not usually included under this heading, as chronic compression takes a unique pathophysiological course.
Palmitoylethanolamide (PEA) is an endogenous fatty acid amide, and lipid modulator PEA has been studied in in vitro and in vivo systems using exogenously added or dosed compound; there is evidence that it binds to a nuclear receptor, through which it exerts a variety of biological effects, some related to chronic inflammation and pain.
Chemotherapy-induced peripheral neuropathy (CIPN) is a nerve-damaging side effect of antineoplastic agents in the common cancer treatment, chemotherapy. CIPN afflicts between 30% and 40% of patients undergoing chemotherapy. Antineoplastic agents in chemotherapy are designed to eliminate rapidly dividing cancer cells, but they can also damage healthy structures, including the peripheral nervous system. CIPN involves various symptoms such as tingling, pain, and numbness in the hands and feet. These symptoms can impair activities of daily living, such as typing or dressing, reduce balance, and increase risk of falls and hospitalizations. They can also give cause to reduce or discontinue chemotherapy. Researchers have conducted clinical trials and studies to uncover the various symptoms, causes, pathogenesis, diagnoses, risk factors, and treatments of CIPN.
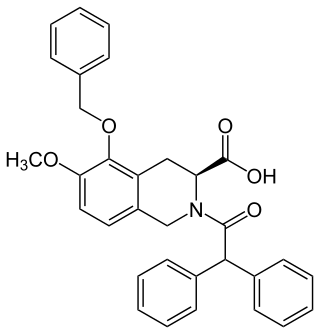
EMA401 is a drug under development for the treatment of peripheral neuropathic pain. Trials were discontinued in 2015, with new trials scheduled to begin March, 2018. It was initially established as a potential drug option for patients suffering pain caused by postherpetic neuralgia. It may also be useful for treating various types of chronic neuropathic pain EMA401 has shown efficacy in preclinical models of shingles, diabetes, osteoarthritis, HIV and chemotherapy. EMA401 is a competitive antagonist of angiotensin II type 2 receptor (AT2R) being developed by the Australian biotechnology company Spinifex Pharmaceuticals. EMA401 target angiotensin II type 2 receptors, which may have importance for painful sensitisation.
Ocular neuropathic pain is a spectrum of disorders of ocular pain which are caused by damage or disease affecting the nerves. Ocular neuropathic pain is frequently associated with damaged or dysfunctional corneal nerves, but the condition can also be caused by peripheral or centralized sensitization. The condition shares some characteristics with somatic neuropathic pain in that it is similarly associated with abnormal sensations (dysesthesia) or pain from normally non-painful stimuli (allodynia), but until recent years has been poorly understood by the medical community, and frequently dismissed by ophthalmologists who were not trained to identify neuropathic pain as a source of unexplained eye pain beyond objective findings noted on slit-lamp examination.
Peripheral mononeuropathy is a nerve related disease where a single nerve, that is used to transport messages from the brain to the peripheral body, is diseased or damaged. Peripheral neuropathy is a general term that indicates any disorder of the peripheral nervous system. The name of the disorder itself can be broken down in order to understand this better; peripheral: in regard to peripheral neuropathy, refers to outside of the brain and spinal cord; neuro: means nerve related; -pathy; means disease. Peripheral mononeuropathy is a disorder that links to Peripheral Neuropathy, as it only effects a single peripheral nerve rather than several damaged or diseased nerves throughout the body. Healthy peripheral nerves are able to “carry messages from the brain and spinal cord to muscles, organs, and other body tissues”.
Vasculitic neuropathy is a peripheral neuropathic disease. In a vasculitic neuropathy there is damage to the vessels that supply blood to the nerves. It can be as part of a systemic problem or can exist as a single-organ issue only affecting the peripheral nervous system (PNS). It is diagnosed with the use of electrophysiological testing, blood tests, nerve biopsy and clinical examination. It is a serious medical condition that can cause prolonged morbidity and disability and generally requires treatment. Treatment depends on the type but it is mostly with corticosteroids or immunomodulating therapies.











