Related Research Articles
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.

In biochemistry, medicine, and related sciences, inositol generally refers to myo-inositol, the most important stereoisomer of the chemical compound cyclohexane-1,2,3,4,5,6-hexol. Its formula is C6H12O6; the molecule has a ring of six carbon atoms, each with an hydrogen atom and a hydroxyl group (–OH). In myo-inositol, two of the hydroxyls, neither adjacent not opposite, lie above the respective hydrogens relative to the mean plane of the ring.

Phosphatidylinositol or inositol phospholipid is a biomolecule. It was initially called "inosite" when it was discovered by Léon Maquenne and Johann Joseph von Scherer in the late 19th century. It was discovered in bacteria but later also found in eukaryotes, and was found to be a signaling molecule.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.

Inositol phosphates are a group of mono- to hexaphosphorylated inositols. Each form of inositol phosphate is distinguished by the number and position of the phosphate group on the inositol ring.
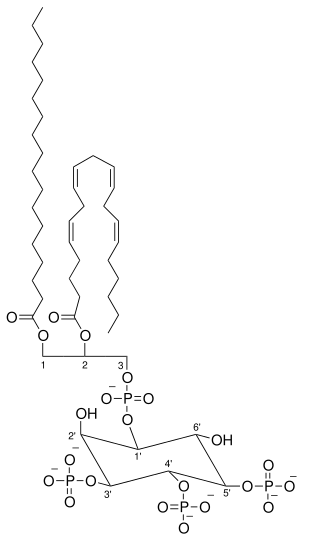
Phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), abbreviated PIP3, is the product of the class I phosphoinositide 3-kinases' (PI 3-kinases) phosphorylation of phosphatidylinositol (4,5)-bisphosphate (PIP2). It is a phospholipid that resides on the plasma membrane.

Phosphatidylinositol (3,4)-bisphosphate is a minor phospholipid component of cell membranes, yet an important second messenger. The generation of PtdIns(3,4)P2 at the plasma membrane activates a number of important cell signaling pathways.

The enzyme Inositol phosphate-phosphatase is of the phosphodiesterase family of enzymes. It is involved in the phosphophatidylinositol signaling pathway, which affects a wide array of cell functions, including but not limited to, cell growth, apoptosis, secretion, and information processing. Inhibition of inositol monophosphatase may be key in the action of lithium in treating bipolar disorder, specifically manic depression.

Inositol (1,4,5) trisphosphate 3-kinase (EC 2.7.1.127), abbreviated here as ITP3K, is an enzyme that facilitates a phospho-group transfer from adenosine triphosphate to 1D-myo-inositol 1,4,5-trisphosphate. This enzyme belongs to the family of transferases, specifically those transferring phosphorus-containing groups (phosphotransferases) with an alcohol group as acceptor. The systematic name of this enzyme class is ATP:1D-myo-inositol-1,4,5-trisphosphate 3-phosphotransferase. ITP3K catalyzes the transfer of the gamma-phosphate from ATP to the 3-position of inositol 1,4,5-trisphosphate to form inositol 1,3,4,5-tetrakisphosphate. ITP3K is highly specific for the 1,4,5-isomer of IP3, and it exclusively phosphorylates the 3-OH position, producing Ins(1,3,4,5)P4, also known as inositol tetrakisphosphate or IP4.

Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase 1(SHIP1) is an enzyme with phosphatase activity. SHIP1 is structured by multiple domain and is encoded by the INPP5D gene in humans. SHIP1 is expressed predominantly by hematopoietic cells but also, for example, by osteoblasts and endothelial cells. This phosphatase is important for the regulation of cellular activation. Not only catalytic but also adaptor activities of this protein are involved in this process. Its movement from the cytosol to the cytoplasmic membrane, where predominantly performs its function, is mediated by tyrosine phosphorylation of the intracellular chains of cell surface receptors that SHIP1 binds. Insufficient regulation of SHIP1 leads to different pathologies.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Type I inositol-3,4-bisphosphate 4-phosphatase is an enzyme that in humans is encoded by the INPP4A gene.

Inositol hexakisphosphate kinase 2 is an enzyme that in humans is encoded by the IP6K2 gene.

Type II inositol-1,4,5-trisphosphate 5-phosphatase is an enzyme that in humans is encoded by the INPP5B gene.

Inositol hexakisphosphate kinase 1 is an enzyme that in humans is encoded by the IP6K1 gene.
Inositol-polyphosphate multikinase is an enzyme with systematic name ATP:1D-myo-inositol-1,4,5-trisphosphate 6-phosphotransferase. This enzyme catalyses the following chemical reaction
Inositol-hexakisphosphate kinase is an enzyme with systematic name ATP:1D-myo-inositol-hexakisphosphate 5-phosphotransferase. This enzyme catalyses the following chemical reaction
Phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase is an enzyme with systematic name 1-phosphatidyl-1D-myo-inositol-3,4,5-trisphosphate 5-phosphohydrolase, that has two isoforms: SHIP1 and SHIP2 (INPPL1).

Inositol polyphosphate kinase (IPK) is a family of enzymes that have a similar 3-dimensional structure. All members of the family catalyze the transfer of phosphate groups from ATP to various inositol phosphates. Members of the family include inositol-polyphosphate multikinases, inositol-hexakisphosphate kinases, inositol-trisphosphate 3-kinases, and inositol-pentakisphosphate 2-kinase, which is more distantly related to the others

Dorothea Fiedler is a chemical biologist and also the first female director of the Leibniz-Forschungsinstitut für Molekulare Pharmakologie in Berlin, Germany.
References
- ↑ "CDFD Profile - Rashna Bhandari" . Retrieved 16 July 2016.
- 1 2 3 "Fellow Profile" . Retrieved 16 July 2016.
- ↑ Mallikarjun, Y. (18 February 2015). "Small molecule with a huge potential". The Hindu. Retrieved 21 June 2018.
- ↑ "In media". Archived from the original on 16 August 2016. Retrieved 16 July 2016.