Related Research Articles
Small nuclear RNA (snRNA) is a class of small RNA molecules that are found within the splicing speckles and Cajal bodies of the cell nucleus in eukaryotic cells. The length of an average snRNA is approximately 150 nucleotides. They are transcribed by either RNA polymerase II or RNA polymerase III. Their primary function is in the processing of pre-messenger RNA (hnRNA) in the nucleus. They have also been shown to aid in the regulation of transcription factors or RNA polymerase II,and maintaining the telomeres.

Spinal muscular atrophy (SMA) is a rare neuromuscular disorder that results in the loss of motor neurons and progressive muscle wasting. It is usually diagnosed in infancy or early childhood and if left untreated it is the most common genetic cause of infant death. It may also appear later in life and then have a milder course of the disease. The common feature is progressive weakness of voluntary muscles,with arm,leg and respiratory muscles being affected first. Associated problems may include poor head control,difficulties swallowing,scoliosis,and joint contractures.
Gideon Dreyfuss is an American biochemist,the Isaac Norris Professor of Biochemistry and Biophysics at the University of Pennsylvania School of Medicine,and an investigator of the Howard Hughes Medical Institute. He was elected to the National Academy of Sciences in 2012.
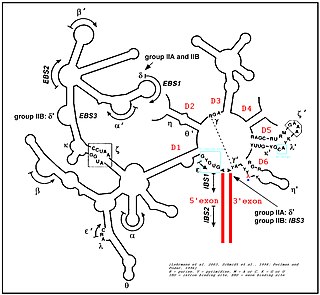
Group II introns are a large class of self-catalytic ribozymes and mobile genetic elements found within the genes of all three domains of life. Ribozyme activity can occur under high-salt conditions in vitro. However,assistance from proteins is required for in vivo splicing. In contrast to group I introns,intron excision occurs in the absence of GTP and involves the formation of a lariat,with an A-residue branchpoint strongly resembling that found in lariats formed during splicing of nuclear pre-mRNA. It is hypothesized that pre-mRNA splicing may have evolved from group II introns,due to the similar catalytic mechanism as well as the structural similarity of the Group II Domain V substructure to the U6/U2 extended snRNA. Finally,their ability to site-specifically insert into DNA sites has been exploited as a tool for biotechnology. For example,group II introns can be modified to make site-specific genome insertions and deliver cargo DNA such as reporter genes or lox sites
An exonic splicing silencer (ESS) is a short region of an exon and is a cis-regulatory element. A set of 103 hexanucleotides known as FAS-hex3 has been shown to be abundant in ESS regions. ESSs inhibit or silence splicing of the pre-mRNA and contribute to constitutive and alternate splicing. To elicit the silencing effect,ESSs recruit proteins that will negatively affect the core splicing machinery.
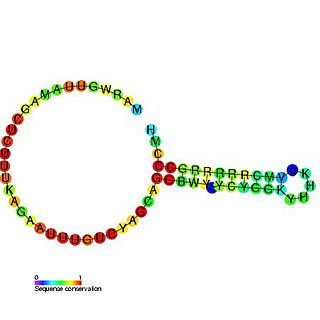
The U7 small nuclear RNA is an RNA molecule and a component of the small nuclear ribonucleoprotein complex. The U7 snRNA is required for histone pre-mRNA processing.

Survival of motor neuron or survival motor neuron (SMN) is a protein that in humans is encoded by the SMN1 and SMN2 genes.

Survival of motor neuron 1 (SMN1),also known as component of gems 1 or GEMIN1,is a gene that encodes the SMN protein in humans.

Gem-associated protein 2 (GEMIN2),also called survival of motor neuron protein-interacting protein 1 (SIP1),is a protein that in humans is encoded by the GEMIN2 gene.

Survival of motor neuron 2 (SMN2) is a gene that encodes the SMN protein in humans.
In molecular biology,exon skipping is a form of RNA splicing used to cause cells to “skip”over faulty or misaligned sections (exons) of genetic code,leading to a truncated but still functional protein despite the genetic mutation.
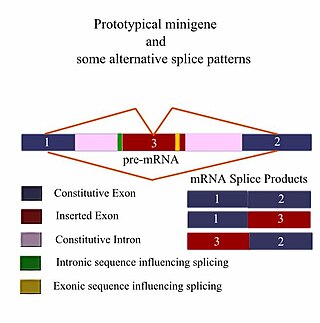
A minigene is a minimal gene fragment that includes an exon and the control regions necessary for the gene to express itself in the same way as a wild type gene fragment. This is a minigene in its most basic sense. More complex minigenes can be constructed containing multiple exons and intron(s). Minigenes provide a valuable tool for researchers evaluating splicing patterns both in vivo and in vitro biochemically assessed experiments. Specifically,minigenes are used as splice reporter vectors and act as a probe to determine which factors are important in splicing outcomes. They can be constructed to test the way both cis-regulatory elements and trans-regulatory elements affect gene expression.

WRAP53 is a gene implicated in cancer development. The name was coined in 2009 to describe the dual role of this gene,encoding both an antisense RNA that regulates the p53 tumor suppressor and a protein involved in DNA repair,telomere elongation and maintenance of nuclear organelles Cajal bodies.

Neurodegenerative diseases are a heterogeneous group of complex disorders linked by the degeneration of neurons in either the peripheral nervous system or the central nervous system. Their underlying causes are extremely variable and complicated by various genetic and/or environmental factors. These diseases cause progressive deterioration of the neuron resulting in decreased signal transduction and in some cases even neuronal death. Peripheral nervous system diseases may be further categorized by the type of nerve cell affected by the disorder. Effective treatment of these diseases is often prevented by lack of understanding of the underlying molecular and genetic pathology. Epigenetic therapy is being investigated as a method of correcting the expression levels of misregulated genes in neurodegenerative diseases.

Nusinersen,marketed as Spinraza,is a medication used in treating spinal muscular atrophy (SMA),a rare neuromuscular disorder. In December 2016,it became the first approved drug used in treating this disorder.

Branaplam is a pyridazine derivative that is being studied as an experimental drug. It was originally developed by Novartis to treat spinal muscular atrophy (SMA);since 2020 it was being developed to treat Huntington's disease but the trial ended in 2023 due to toxicity concerns.

Risdiplam,sold under the brand name Evrysdi,is a medication used to treat spinal muscular atrophy (SMA) and the first oral medication approved to treat this disease.

Alberto Kornblihtt is an Argentine molecular biologist who specializes in alternative ribonucleic acid splicing. During his postdoctoral training with Francisco Baralle in Oxford,Kornblihtt documented one of the first cases of alternative splicing,explaining how a single transcribed gene can generate multiple protein variants. Kornblihtt was elected as a foreign associate of the National Academy of Sciences of the United States in 2011,received the Diamond Award for the most relevant scientist of Argentina of the decade,alongside physicist Juan Martin Maldacena,in 2013,and was incorporated to the Académie des Sciences of France in 2022.
Robin Elizabeth Reed was an American professor of cell biology at the Harvard Medical School. Her research considered the molecular mechanisms that underpin neurodegenerative disease.
Stephen Donald Wilton,also known as Steve Wilton,is an Australian molecular biologist and academic,serving as the Foundation Professor of Molecular Therapy at Murdoch University and adjunct professor at the University of Western Australia (UWA). He also fulfills dual roles as a Director at the Perron Institute for Neurological and Translational Science and deputy director at Murdoch's Centre for Molecular Medicine and Innovative Therapeutics (CMMIT).
References
- 1 2 3 "Iowa State University–Ravindra N Singh".
- 1 2 "Google Scholar–Ravindra Singh".
- ↑ "ISU researchers find possible treatment for Spinal Muscular Atrophy".
- ↑ "First-of-its-Kind Super Minigene to Boost Spinal Muscular Atrophy Research".
- 1 2 "Iowa State University researcher honored at White House ceremony".
- ↑ "At UMass Lab, A Eureka Moment".
- ↑ "Ravindra Singh".
- 1 2 "New Biotechnology Council Members Named".
- 1 2 "Graduate Council–Meeting Attendance 2015-2016".
- ↑ "A reverse transcriptase/maturase promotes splicing by binding at its own coding segment in a group II intron RNA".
- ↑ "Diverse role of survival motor neuron protein".
- ↑ "Splicing of a Critical Exon of Human Survival Motor Neuron Is Regulated by a Unique Silencer Element Located in the Last Intron".
- ↑ "An extended inhibitory context causes skipping of exon 7 of SMN2 in spinal muscular atrophy".
- ↑ "How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy".
- ↑ "Spinal muscular atrophy (SMA) treatment via targeting of SMN2 splice site inhibitory sequences".
- ↑ "Spinal muscular atrophy treatment via targeting smn2 catalytic core".
- 1 2 "A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy".
- ↑ "In vivo selection reveals combinatorial controls that define a critical exon in the spinal muscular atrophy genes".
- ↑ "Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes".
- ↑ "TIA1 Prevents Skipping of a Critical Exon Associated with Spinal Muscular Atrophy".
- ↑ "A multi-exon-skipping detection assay reveals surprising diversity of splice isoforms of spinal muscular atrophy genes".
- ↑ "An intronic structure enabled by a long-distance interaction serves as a novel target for splicing correction in spinal muscular atrophy".
- ↑ "Severe impairment of male reproductive organ development in a low SMN expressing mouse model of spinal muscular atrophy".
- ↑ "Activation of a cryptic 5' splice site reverses the impact of pathogenic splice site mutations in the spinal muscular atrophy gene".
- ↑ "High-affinity RNA targets of the Survival Motor Neuron protein reveal diverse preferences for sequence and structural motifs".
- ↑ "Human Survival Motor Neuron genes generate a vast repertoire of circular RNAs".
- ↑ "Transcriptome- and proteome-wide effects of a circular RNA encompassing four early exons of the spinal muscular atrophy genes".
- ↑ "High Concentration of an ISS-N1-Targeting Antisense Oligonucleotide Causes Massive Perturbation of the Transcriptome".
- ↑ "Diverse targets of SMN2-directed splicing-modulating small molecule therapeutics for spinal muscular atrophy".
- ↑ "A super minigene with a short promoter and truncated introns recapitulates essential features of transcription and splicing regulation of the SMN1 and SMN2 genes".