See also
 recognition sequence (P4863) (see uses )
recognition sequence (P4863) (see uses )
- DNA-binding domain
- Transcription factor#Classes, for more examples
A recognition sequence is a DNA sequence to which a structural motif of a DNA-binding domain exhibits binding specificity. Recognition sequences are palindromes. [1]
The transcription factor Sp1 for example, binds the sequences 5'-(G/T)GGGCGG(G/A)(G/A)(C/T)-3', where (G/T) indicates that the domain will bind a guanine or thymine at this position.
The restriction endonuclease PstI recognizes, binds, and cleaves the sequence 5'-CTGCAG-3'.
A recognition sequence is different from a recognition site . A given recognition sequence can occur one or more times, or not at all, on a specific DNA fragment. A recognition site is specified by the position of the site. For example, there are two PstI recognition sites in the following DNA sequence fragment, starting at base 9 and 31 respectively. A recognition sequence is a specific sequence, usually very short (less than 10 bases). Depending on the degree of specificity of the protein, a DNA-binding protein can bind to more than one specific sequence. For PstI, which has a single sequence specificity, it is 5'-CTGCAG-3'. It is always the same whether at the first recognition site or the second in the following example sequence. For Sp1, which has multiple (16) sequence specificity as shown above, the two recognition sites in the following example sequence fragment are at 18 and 32, and their respective recognition sequences are 5'-GGGGCGGAGC-3' and 5'-TGGGCGGAAC-3'.
5'-AACGTTAGCTGCAGTCGGGGCGGAGCTAGGCTGCAGGAATTGGGCGGAACCT-3'

In molecular biology, a transcription factor (TF) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The function of TFs is to regulate—turn on and off—genes in order to make sure that they are expressed in the desired cells at the right time and in the right amount throughout the life of the cell and the organism. Groups of TFs function in a coordinated fashion to direct cell division, cell growth, and cell death throughout life; cell migration and organization during embryonic development; and intermittently in response to signals from outside the cell, such as a hormone. There are approximately 1600 TFs in the human genome. Transcription factors are members of the proteome as well as regulome.
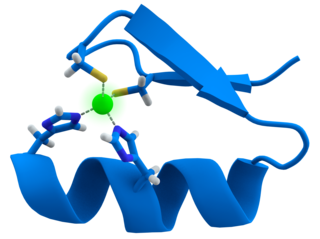
A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. It was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (Xenopus laevis) transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. Xenopus laevis TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in Drosophila. It often appears as a metal-binding domain in multi-domain proteins.
In molecular biology and genetics, transcriptional regulation is the means by which a cell regulates the conversion of DNA to RNA (transcription), thereby orchestrating gene activity. A single gene can be regulated in a range of ways, from altering the number of copies of RNA that are transcribed, to the temporal control of when the gene is transcribed. This control allows the cell or organism to respond to a variety of intra- and extracellular signals and thus mount a response. Some examples of this include producing the mRNA that encode enzymes to adapt to a change in a food source, producing the gene products involved in cell cycle specific activities, and producing the gene products responsible for cellular differentiation in multicellular eukaryotes, as studied in evolutionary developmental biology.

In biochemistry, a nuclease is an enzyme capable of cleaving the phosphodiester bonds that link nucleotides together to form nucleic acids. Nucleases variously affect single and double stranded breaks in their target molecules. In living organisms, they are essential machinery for many aspects of DNA repair. Defects in certain nucleases can cause genetic instability or immunodeficiency. Nucleases are also extensively used in molecular cloning.

DNA-binding proteins are proteins that have DNA-binding domains and thus have a specific or general affinity for single- or double-stranded DNA. Sequence-specific DNA-binding proteins generally interact with the major groove of B-DNA, because it exposes more functional groups that identify a base pair.

EGR-1 or NGFI-A is a protein that in humans is encoded by the EGR1 gene.

In molecular genetics, a repressor is a DNA- or RNA-binding protein that inhibits the expression of one or more genes by binding to the operator or associated silencers. A DNA-binding repressor blocks the attachment of RNA polymerase to the promoter, thus preventing transcription of the genes into messenger RNA. An RNA-binding repressor binds to the mRNA and prevents translation of the mRNA into protein. This blocking or reducing of expression is called repression.

A leucine zipper is a common three-dimensional structural motif in proteins. They were first described by Landschulz and collaborators in 1988 when they found that an enhancer binding protein had a very characteristic 30-amino acid segment and the display of these amino acid sequences on an idealized alpha helix revealed a periodic repetition of leucine residues at every seventh position over a distance covering eight helical turns. The polypeptide segments containing these periodic arrays of leucine residues were proposed to exist in an alpha-helical conformation and the leucine side chains from one alpha helix interdigitate with those from the alpha helix of a second polypeptide, facilitating dimerization.
A DNA-binding domain (DBD) is an independently folded protein domain that contains at least one structural motif that recognizes double- or single-stranded DNA. A DBD can recognize a specific DNA sequence or have a general affinity to DNA. Some DNA-binding domains may also include nucleic acids in their folded structure.

The TATA-binding protein (TBP) is a general transcription factor that binds to a DNA sequence called the TATA box. This DNA sequence is found about 30 base pairs upstream of the transcription start site in some eukaryotic gene promoters.

Transcription factor Sp1, also known as specificity protein 1* is a protein that in humans is encoded by the SP1 gene.
Therapeutic gene modulation refers to the practice of altering the expression of a gene at one of various stages, with a view to alleviate some form of ailment. It differs from gene therapy in that gene modulation seeks to alter the expression of an endogenous gene whereas gene therapy concerns the introduction of a gene whose product aids the recipient directly.
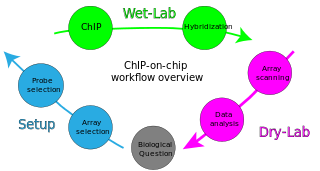
ChIP-on-chip is a technology that combines chromatin immunoprecipitation ('ChIP') with DNA microarray ("chip"). Like regular ChIP, ChIP-on-chip is used to investigate interactions between proteins and DNA in vivo. Specifically, it allows the identification of the cistrome, the sum of binding sites, for DNA-binding proteins on a genome-wide basis. Whole-genome analysis can be performed to determine the locations of binding sites for almost any protein of interest. As the name of the technique suggests, such proteins are generally those operating in the context of chromatin. The most prominent representatives of this class are transcription factors, replication-related proteins, like origin recognition complex protein (ORC), histones, their variants, and histone modifications.

Nuclear transcription factor Y subunit alpha is a protein that in humans is encoded by the NFYA gene.

Transcription factor Sp2 is a protein that in humans is encoded by the SP2 gene.
Zinc finger protein chimera are chimeric proteins composed of a DNA-binding zinc finger protein domain and another domain through which the protein exerts its effect. The effector domain may be a transcriptional activator (A) or repressor (R), a methylation domain (M) or a nuclease (N).
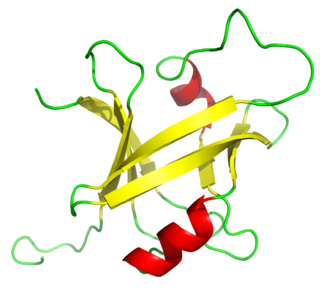
The B3 DNA binding domain (DBD) is a highly conserved domain found exclusively in transcription factors combined with other domains. It consists of 100-120 residues, includes seven beta strands and two alpha helices that form a DNA-binding pseudobarrel protein fold ; it interacts with the major groove of DNA.

Pho4 is a protein with a basic helix-loop-helix (bHLH) transcription factor. It is found in S. cerevisiae and other yeasts. It functions as a transcription factor to regulate phosphate responsive genes located in yeast cells. The Pho4 protein homodimer is able to do this by binding to DNA sequences containing the bHLH binding site 5'-CACGTG-3'. This sequence is found in the promoters of genes up-regulated in response to phosphate availability such as the PHO5 gene.

DNA binding sites are a type of binding site found in DNA where other molecules may bind. DNA binding sites are distinct from other binding sites in that (1) they are part of a DNA sequence and (2) they are bound by DNA-binding proteins. DNA binding sites are often associated with specialized proteins known as transcription factors, and are thus linked to transcriptional regulation. The sum of DNA binding sites of a specific transcription factor is referred to as its cistrome. DNA binding sites also encompasses the targets of other proteins, like restriction enzymes, site-specific recombinases and methyltransferases.
PstI is a type II restriction endonuclease isolated from the Gram negative species, Providencia stuartii.