
Soap is a salt of a fatty acid used for cleaning and lubricating products as well as other applications. In a domestic setting, soaps, specifically "toilet soaps", are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, emulsifiers, and catalysts.

A resin is a solid or highly viscous liquid that can be converted into a polymer. Resins may be biological or synthetic in origin, but are typically harvested from plants. Resins are mixtures of organic compounds, and predominantly terpenes. Well known resins include amber, hashish, frankincense, myrrh and the animal-derived resin, shellac. Resins are commonly used in varnishes, adhesives, food additives, incenses and perfumes.

Turpentine is a fluid obtained by the distillation of resin harvested from living trees, mainly pines. Principally used as a specialized solvent, it is also a source of material for organic syntheses.

Varnish is a clear transparent hard protective coating or film. It is not to be confused with wood stain. It usually has a yellowish shade due to the manufacturing process and materials used, but it may also be pigmented as desired. It is sold commercially in various shades.
Saponification is a process of cleaving esters into carboxylate salts and alcohols by the action of aqueous alkali. Typically aqueous sodium hydroxide solutions are used. It is an important type of alkaline hydrolysis. When the carboxylate is long chain, its salt is called a soap. The saponification of ethyl acetate gives sodium acetate and ethanol:
Rosin, also known as colophony or Greek pitch (Latin: pix graeca), is a resinous material obtained from pine trees and other plants, mostly conifers. The primary components of rosin are diterpenoids, i.e., C20 carboxylic acids. Rosin consists mainly of resin acids, especially abietic acid. Rosin often appears as a semi-transparent, brittle substance that ranges in color from yellow to black and melts at stove-top temperatures.

Abietic acid is a diterpenoid found in coniferous trees. It is supposed to exist as a defend the host plant from insect attack or various wounds. Chemically, it is a complicated molecule featuring two alkene groups and a carboxylic acid within a chiral tricyclic framework. As the major component of rosin, it is a commercially important. Historically speaking, it was a major component of naval stores. It is the most common of the resin acids. Another common resin acid is pimaric acid, which converts to abietic acid upon heating.

The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chips with a hot mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S), known as white liquor, that breaks the bonds that link lignin, hemicellulose, and cellulose. The technology entails several steps, both mechanical and chemical. It is the dominant method for producing paper. In some situations, the process has been controversial because kraft plants can release odorous products and in some situations produce substantial liquid wastes.

Pinus roxburghii, commonly known as chir pine or longleaf Indian pine, is a species of pine tree native to the Himalayas. It was named after William Roxburgh.

Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins, but is increasingly being accomplished using nanofiltration or reverse osmosis membranes.
Resin acid refers to any of several related carboxylic acids found in tree resins. Nearly all resin acids have the same basic skeleton: three fused rings having the empirical formula C19H29COOH. Resin acids occur in nature as tacky, yellowish gums consisting of several compounds. They are water-insoluble. A common resin acid is abietic acid. Resin acids are used to produce soaps for diverse applications, but their use is being displaced increasingly by synthetic acids such as 2-ethylhexanoic acid or petroleum-derived naphthenic acids.

In industrial chemistry, black liquor is the by-product from the kraft process when digesting pulpwood into paper pulp removing lignin, hemicelluloses and other extractives from the wood to free the cellulose fibers.
Tall oil, also called liquid rosin or tallol, is a viscous yellow-black odorous liquid obtained as a by-product of the kraft process of wood pulp manufacture when pulping mainly coniferous trees. The name originated as an anglicization of the Swedish tallolja. Tall oil is the third largest chemical by-product in a kraft mill after lignin and hemicellulose; the yield of crude tall oil from the process is in the range of 30–50 kg / ton pulp. It may contribute to 1.0–1.5% of the mill's revenue if not used internally.
Lignosulfonates (LS) are water-soluble anionic polyelectrolyte polymers: they are byproducts from the production of wood pulp using sulfite pulping. Most delignification in sulfite pulping involves acidic cleavage of ether bonds, which connect many of the constituents of lignin. Sulfonated lignin (SL) refers to other forms of lignin by-product, such as those derived from the much more popular Kraft process, that have been processed to add sulfonic acid groups. The two have similar uses and are commonly confused with each other, with SL being much cheaper. LS and SL both appear as free-flowing powders; the former is light brown while the latter is dark brown.
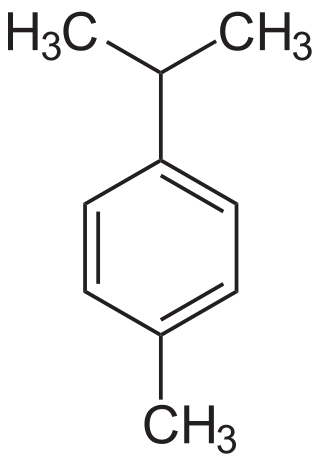
Naval stores refers to the industry that produces rosin, turpentine, tall oil, pine oil, and other oleoresin collected from conifers. The term was originally applied to the compounds used in building and maintaining wooden sailing ships. Presently, pine compounds produced by the naval stores industry are used to manufacture soap, paint, varnish, shoe polish, lubricants, linoleum, and roofing materials.
In industrial paper-making processes, organosolv is a pulping technique that uses an organic solvent to solubilise lignin and hemicellulose. It has been considered in the context of both pulp and paper manufacture and biorefining for subsequent conversion of cellulose to fuel ethanol. The process was invented by Theodor Kleinert in 1968 as an environmentally benign alternative to kraft pulping.
Lithium 12-hydroxystearate (C18H35LiO3) is a chemical compound classified as a lithium soap. In chemistry, "soap" refers to salts of fatty acids. Lithium 12-hydroxystearate is a white solid. Lithium soaps are key component of many lubricating greases.

Levopimaric acid is an abietane-type of diterpene resin acid. It is a major constituent of pine oleoresin with the chemical formula of C20H30O2. In general, the abietene types of diterpene resin acid have various biological activities, such as antibacterial, cardiovascular and antioxidant. Levopimaric acid accounts for about 18 to 25% of pine oleoresin. The production of oleoresin by conifer species is an important component of the defense response against insect attack and fungal pathogen infection.
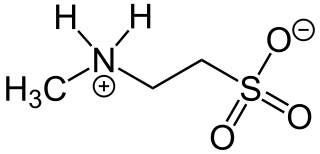
N-Methyltaurine is an aminosulfonic acid which is present as a zwitterion in the crystalline state and in polar solvents. In contrast to the widespread taurine, N-methyltaurine has been found in nature only in red algae, where it is formed by methylation of taurine. It is suitable for esterification with long-chain carboxylic acids to taurides (acylaminoethansulfonaten) because of its high polarity and the relatively good solubility of its alkaline earth metal salts, which are also used as mild anionic surfactants.











