Related Research Articles
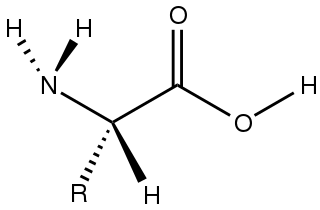
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most prevalent are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code.
Combinatorial chemistry comprises chemical synthetic methods that make it possible to prepare a large number of compounds in a single process. These compound libraries can be made as mixtures, sets of individual compounds or chemical structures generated by computer software. Combinatorial chemistry can be used for the synthesis of small molecules and for peptides.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Medicinal chemistry is discipline at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents, or bio-active molecules (drugs).

Robert Bruce Merrifield was an American biochemist who won the Nobel Prize in Chemistry in 1984 for the invention of solid phase peptide synthesis.
In chemistry, solid-phase synthesis is a method in which molecules are covalently bound on a solid support material and synthesised step-by-step in a single reaction vessel utilising selective protecting group chemistry. Benefits compared with normal synthesis in a liquid state include:
Chemical biology is a scientific discipline spanning the fields of chemistry and biology. The discipline involves the application of chemical techniques, analysis, and often small molecules produced through synthetic chemistry, to the study and manipulation of biological systems. In contrast to biochemistry, which involves the study of the chemistry of biomolecules and regulation of biochemical pathways within and between cells, chemical biology deals with chemistry applied to biology.

Aptamers are short sequences of artificial DNA or RNA that bind a specific target molecule. Like antibodies, which are used for similar purposes in biotechnology and medicine, they can show strong binding to their target, with little or no off-target binding. To highlight how they are similar to and different from antibodies, aptamers are sometimes called chemical antibodies. Aptamers and antibodies can be used in many of the same tasks, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes.

mRNA display is a display technique used for in vitro protein, and/or peptide evolution to create molecules that can bind to a desired target. The process results in translated peptides or proteins that are associated with their mRNA progenitor via a puromycin linkage. The complex then binds to an immobilized target in a selection step. The mRNA-protein fusions that bind well are then reverse transcribed to cDNA and their sequence amplified via a polymerase chain reaction. The result is a nucleotide sequence that encodes a peptide with high affinity for the molecule of interest.
William F. "Bill" DeGrado is the Professor of Pharmaceutical Chemistry at the University of California, San Francisco (UCSF) and a member of the National Academy of Sciences.

HIV-1 protease (PR) is a retroviral aspartyl protease (retropepsin), an enzyme involved with peptide bond hydrolysis in retroviruses, that is essential for the life-cycle of HIV, the retrovirus that causes AIDS. HIV protease cleaves newly synthesized polyproteins at nine cleavage sites to create the mature protein components of an HIV virion, the infectious form of a virus outside of the host cell. Without effective HIV protease, HIV virions remain uninfectious.
Kim D. Janda is an American chemist who studies on medicinal chemistry, molecular biology, immunology and neuropharmacology.

Demoxytocin (INN), also known as desaminooxytocin or deaminooxytocin, as well as 1-(3-mercaptopropanoic acid)oxytocin ([Mpa1]OT), is an oxytocic peptide drug that is used to induce labor, promote lactation, and to prevent and treat puerperal (postpartum) mastitis. Demoxytocin is a synthetic analogue of oxytocin and has similar activities, but is more potent and has a longer half-life in comparison. Unlike oxytocin, which is given via intravenous injection, demoxytocin is administered as a buccal tablet formulation.
Morten P. Meldal is a Danish chemist. He is a professor of Chemistry at University of Copenhagen in Copenhagen, Denmark. He is best known for developing the CuAAC-click reaction, concurrent with but independent of Valery V. Fokin and K. Barry Sharpless.
DNA-encoded chemical libraries (DEL) is a technology for the synthesis and screening on unprecedented scale of collections of small molecule compounds. DEL is used in medicinal chemistry to bridge the fields of combinatorial chemistry and molecular biology. The aim of DEL technology is to accelerate the drug discovery process and in particular early phase discovery activities such as target validation and hit identification.
Ralph Franz Hirschmann was a German American chemist who led a team that was responsible for the first organic synthesis of an enzyme, a ribonuclease.
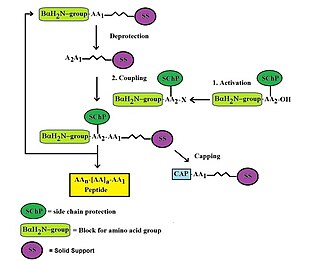
Custom peptide synthesis is the commercial production of peptides for use in biochemistry, biology, biotechnology, pharmacology and molecular medicine. Custom peptide synthesis provides synthetic peptides as valuable tools to biomedical laboratories. Synthetic oligopeptides are used extensively in research for structure-function analysis, for the development of binding assays, the study of receptor agonist/antagonists or as immunogens for the production of specific antibodies. Generally, peptides are synthesized by coupling the carboxyl group or C-terminus of one amino acid to the amino group or N-terminus of another using automated solid phase peptide synthesis chemistries. However, liquid phase synthesis may also be used for specific needs.

Building block is a term in chemistry which is used to describe a virtual molecular fragment or a real chemical compound the molecules of which possess reactive functional groups. Building blocks are used for bottom-up modular assembly of molecular architectures: nano-particles, metal-organic frameworks, organic molecular constructs, supra-molecular complexes. Using building blocks ensures strict control of what a final compound or a (supra)molecular construct will be.
Synthetic biopolymers are human-made copies of biopolymers obtained by abiotic chemical routes. Synthetic biopolymer of different chemical nature have been obtained, including polysaccharides, glycoproteins, peptides and proteins, polyhydroxoalkanoates, polyisoprenes.
Peptide therapeutics are peptides or polypeptides which are used to for the treatment of diseases. Naturally occurring peptides may serve as hormones, growth factors, neurotransmitters, ion channel ligands, and anti-infectives; peptide therapeutics mimic such functions. Peptide Therapeutics are seen as relatively safe and well-tolerated as peptides can be metabolized by the body.
References
- ↑ "Richard A. Houghten Biography". TPIMS. 2008. Archived from the original on 2008-05-16. Retrieved 2008-06-29.
- 1 2 Fischer, Lawrence (1992-10-06). "New Drugs by Process of Elimination". New York Times . Retrieved 2008-06-29.
- ↑ "Management biographies". Mixture Science Incorporated. Archived from the original on 2008-03-29. Retrieved 2008-06-29.
- ↑ "2003 ENTREPRENEUR IN RESIDENCE". Lyles Center. California State University, Fresno. Archived from the original on 2008-05-07. Retrieved 2008-06-29.
- ↑ Nill, Kimball R. (2002). Glossary of Biotechnological Terms. CRC Press. p. 55. ISBN 1-58716-122-2.
- ↑ Seethala, Ramakrishna; Fernandes, Prabhavathi B. (2001). Handbook of Drug Screening. Informa Health Care. pp. 357–383. ISBN 0-8247-0562-9.
- ↑ Houghten, Richard A. (August 1, 1985). "General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids". Proceedings of the National Academy of Sciences . United States National Academy of Sciences. 82 (15): 5131–5135. Bibcode:1985PNAS...82.5131H. doi: 10.1073/pnas.82.15.5131 . PMC 390513 . PMID 2410914.
- ↑ Houghten, R A (August 1985). "General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids". Proceedings of the National Academy of Sciences. 82 (15): 5132–5133. Bibcode:1985PNAS...82.5131H. doi: 10.1073/pnas.82.15.5131 . ISSN 0027-8424. PMC 390513 . PMID 2410914.
- ↑ Houghten, R.A.; Pinilla, Clemencia; Blondelle, Sylvie E.; Appel, Jon R.; Dooley, Colette T.; Cuervo, Julio H. (7 November 1991). "Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery". Nature . 354 (6348): 84–86. Bibcode:1991Natur.354...84H. doi:10.1038/354084a0. PMID 1719428. S2CID 4279178.
- ↑ Houghten, Richard A. (1994). "Soluble combinatorial libraries: Extending the range and repertoire of chemical diversity". Methods. 6 (4): 354–360. doi:10.1006/meth.1994.1035.
- ↑ ""Classical" Papers in Molecular Diversity and Solid Phase Synthesis". CSPS Pharmaceuticals, Inc. Retrieved 2008-06-28.
- 1 2 "Awards". Archived from the original on 2008-05-17. Retrieved 2008-07-14.
- ↑ "Ralph Hirschmann Award in Peptide Chemistry Recipients". American Chemical Society. Archived from the original on 16 August 2022. Retrieved 16 August 2022.
- ↑ "AAPS FELLOWS" (PDF). American Association of Pharmaceutical Scientists. Archived (PDF) from the original on 23 February 2022. Retrieved 16 August 2022.
- ↑ "IRSC Foundation - Community Programs". IRSC Foundation. Indian River State College. Archived from the original on 4 March 2021. Retrieved 16 August 2022.