Related Research Articles

Photosynthesis is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. Photosynthesis usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth.
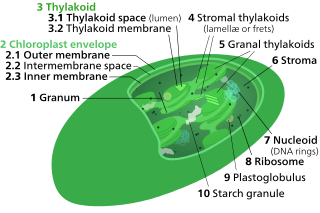
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as grana. Grana are connected by intergranal or stromal thylakoids, which join granum stacks together as a single functional compartment.

Chloroflexus aurantiacus is a photosynthetic bacterium isolated from hot springs, belonging to the green non-sulfur bacteria. This organism is thermophilic and can grow at temperatures from 35 to 70 °C. Chloroflexus aurantiacus can survive in the dark if oxygen is available. When grown in the dark, Chloroflexus aurantiacus has a dark orange color. When grown in sunlight it is dark green. The individual bacteria tend to form filamentous colonies enclosed in sheaths, which are known as trichomes.

Photosystems are functional and structural units of protein complexes involved in photosynthesis. Together they carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons. Photosystems are found in the thylakoid membranes of plants, algae, and cyanobacteria. These membranes are located inside the chloroplasts of plants and algae, and in the cytoplasmic membrane of photosynthetic bacteria. There are two kinds of photosystems: PSI and PSII.

Photosystem I is one of two photosystems in the photosynthetic light reactions of algae, plants, and cyanobacteria. Photosystem I is an integral membrane protein complex that uses light energy to catalyze the transfer of electrons across the thylakoid membrane from plastocyanin to ferredoxin. Ultimately, the electrons that are transferred by Photosystem I are used to produce the moderate-energy hydrogen carrier NADPH. The photon energy absorbed by Photosystem I also produces a proton-motive force that is used to generate ATP. PSI is composed of more than 110 cofactors, significantly more than Photosystem II.

Hartmut Michel is a German biochemist, who received the 1988 Nobel Prize in Chemistry for determination of the first crystal structure of an integral membrane protein, a membrane-bound complex of proteins and co-factors that is essential to photosynthesis.

Purple bacteria or purple photosynthetic bacteria are Gram-negative proteobacteria that are phototrophic, capable of producing their own food via photosynthesis. They are pigmented with bacteriochlorophyll a or b, together with various carotenoids, which give them colours ranging between purple, red, brown, and orange. They may be divided into two groups – purple sulfur bacteria and purple non-sulfur bacteria. Purple bacteria are anoxygenic phototrophs widely spread in nature, but especially in aquatic environments, where there are anoxic conditions that favor the synthesis of their pigments.
A photosynthetic reaction center is a complex of several proteins, biological pigments, and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules (also named chromophores or pigments) such as chlorophyll and pheophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used, via a chain of nearby electron acceptors, for a transfer of hydrogen atoms (as protons and electrons) from H2O or hydrogen sulfide towards carbon dioxide, eventually producing glucose. These electron transfer steps ultimately result in the conversion of the energy of photons to chemical energy.
A light-harvesting complex consists of a number of chromophores which are complex subunit proteins that may be part of a larger super complex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. The light which is captured by the chromophores is capable of exciting molecules from their ground state to a higher energy state, known as the excited state. This excited state does not last very long and is known to be short-lived.

The Fenna–Matthews–Olson (FMO) complex is a water-soluble complex and was the first pigment-protein complex (PPC) to be structure analyzed by x-ray spectroscopy. It appears in green sulfur bacteria and mediates the excitation energy transfer from light-harvesting chlorosomes to the membrane-embedded bacterial reaction center (bRC). Its structure is trimeric (C3-symmetry). Each of the three monomers contains eight bacteriochlorophyll a molecules. They are bound to the protein scaffold via chelation of their central magnesium atom either to amino acids of the protein or water-bridged oxygen atoms.
Quantum biology is the study of applications of quantum mechanics and theoretical chemistry to aspects of biology that cannot be accurately described by the classical laws of physics. An understanding of fundamental quantum interactions is important because they determine the properties of the next level of organization in biological systems.
The following outline is provided as an overview of and topical guide to cell biology:

Photosynthetic reaction centre proteins are main protein components of photosynthetic reaction centres (RCs) of bacteria and plants. They are transmembrane proteins embedded in the chloroplast thylakoid or bacterial cell membrane.

The antenna complex in purple photosynthetic bacteria are protein complexes responsible for the transfer of solar energy to the photosynthetic reaction centre. Purple bacteria, particularly Rhodopseudomonas acidophila of purple non-sulfur bacteria, have been one of the main groups of organisms used to study bacterial antenna complexes so much is known about this group's photosynthetic components. It is one of the many independent types of light-harvesting complex used by various photosynthetic organisms.
Graham R. Fleming is a professor of chemistry at the University of California, Berkeley and member of the Kavli Energy NanoScience Institute based at UCB.

Light-dependent reactions are certain photochemical reactions involved in photosynthesis, the main process by which plants acquire energy. There are two light dependent reactions: the first occurs at photosystem II (PSII) and the second occurs at photosystem I (PSI).
Roseiflexus castenholzii is a heterotrophic, thermophilic, filamentous anoxygenetic phototroph (FAP) bacterium. This species is in one of two genera of FAPs that lack chlorosomes. R. castenholzii was first isolated from red-colored bacterial mats located Nakabusa hot springs in Japan. Because this organism is a phototroph, it utilizes photosynthesis to fix carbon dioxide and build biomolecules. R. castenholzii has three photosynthetic complexes: light-harvesting only, reaction center only, and light-harvesting with reaction center.

Alfred William Rutherford is Professor and Chair in Biochemistry of Solar energy in the Department of Life sciences at Imperial College London.
Klaus Schulten was a German-American computational biophysicist and the Swanlund Professor of Physics at the University of Illinois at Urbana-Champaign. Schulten used supercomputing techniques to apply theoretical physics to the fields of biomedicine and bioengineering and dynamically model living systems. His mathematical, theoretical, and technological innovations led to key discoveries about the motion of biological cells, sensory processes in vision, animal navigation, light energy harvesting in photosynthesis, and learning in neural networks.
Leonid A. Sazanov is a professor at the Institute of Science and Technology Austria (ISTA). Sazanov research explores the structure and function of large membrane protein complexes from the domain of bioenergetics. These molecular machines interconvert redox energy and proton motive force across biological membranes using a variety of mechanisms.
References
- ↑ Cogdell, Richard. "School of Molecular Biosciences". University of Glasgow. Retrieved 2 March 2023.
- ↑ "Society Fellows among new BBSRC Council members". Royal Society of Biology. Retrieved 2 March 2023.
- ↑ McDermott, G.; Prince, S. M.; Freer, A. A.; Hawthornthwaite-Lawless, A. M.; Papiz, M. Z.; Cogdell, R. J.; Isaacs, N. W. (April 1995). "Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria". Nature. 374 (6522): 517–521. Bibcode:1995Natur.374..517M. doi:10.1038/374517a0. S2CID 4258914.
- ↑ "Synthetic biology and industrial biotech". University of Glasgow. Retrieved 2 March 2023.
- ↑ Cogdell, Richard (January 2019). "Editorial". Journal of the Royal Society Interface. 16 (150): 20190016. doi:10.1098/rsif.2019.0016. PMC 6364651 . PMID 30958177.
- ↑ Cogdell, Richard. "Fellow detail page". The Royal Society. Retrieved 2 March 2023.
- ↑ Cogdell, Richard. "Fellowship". Royal Society of Edinburgh. Retrieved 2 March 2023.
- ↑ "DAP - fact sheet" (PDF). Daiwa Anglo-Japanese Foundation. Retrieved 2 March 2023.