
A diaper or a nappy is a type of underwear that allows the wearer to urinate or defecate without using a toilet, by absorbing or containing waste products to prevent soiling of outer clothing or the external environment. When diapers become wet or soiled, they require changing, generally by a second person such as a parent or caregiver. Failure to change a diaper on a sufficiently regular basis can result in skin problems around the area covered by the diaper.

Kleenex is a brand name primarily known for their line of facial tissues. Often used informally as a genericized trademark for facial tissue, Kleenex is a registered trademark of Kimberly-Clark applied to products made in 78 countries. The brand has other paper products like napkins and toilet roll.

Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer synthesized in 1962 by Dr. Donald J. Connolly at the DuPont Experimental Station in Wilmington Delaware. Additional work on the polymer family was performed in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ionic properties that are called ionomers. Nafion's unique ionic properties are a result of incorporating perfluorovinyl ether groups terminated with sulfonate groups onto a tetrafluoroethylene (PTFE) backbone. Nafion has received a considerable amount of attention as a proton conductor for proton exchange membrane (PEM) fuel cells because of its excellent chemical and mechanical stability in the harsh conditions of this application.
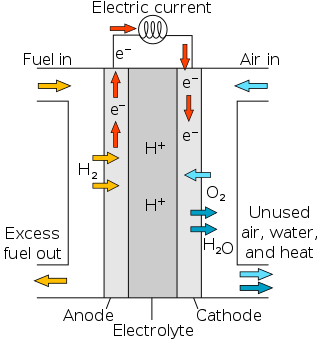
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.

Pampers is an American brand for babies and toddlers products marketed by Procter & Gamble. This includes diapers, wipes and etc.

Huggies is an American company that sells disposable diapers and baby wipes that is marketed by Kimberly-Clark. Huggies were first test marketed in 1968, then introduced to the public in 1977 to replace the Kimbies brand.

An ionomer is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent are ionized. The ionized units are often carboxylic acid groups.

Goodnites are diapers designed for managing bedwetting. Goodnites are produced by Kimberly-Clark. The product has also been seen titled as Huggies Goodnites on official Huggies branded webpages.
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen gas. This is their essential function when incorporated into a membrane electrode assembly (MEA) of a proton-exchange membrane fuel cell or of a proton-exchange membrane electrolyser: separation of reactants and transport of protons while blocking a direct electronic pathway through the membrane.

Sodium polyacrylate (ACR, ASAP, or PAAS), also known as waterlock, is a sodium salt of polyacrylic acid with the chemical formula [−CH2−CH(CO2Na)−]n and has broad applications in consumer products. This super-absorbent polymer (SAP) has the ability to absorb 100 to 1000 times its mass in water. Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain. It is a polymer made up of chains of acrylate compounds. It contains sodium, which gives it the ability to absorb large amounts of water. When dissolved in water, it forms a thick and transparent solution due to the ionic interactions of the molecules. Sodium polyacrylate has many favorable mechanical properties. Some of these advantages include good mechanical stability, high heat resistance, and strong hydration.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.

Nonwoven fabric or non-woven fabric is a fabric-like material made from staple fibre (short) and long fibres, bonded together by chemical, mechanical, heat or solvent treatment. The term is used in the textile manufacturing industry to denote fabrics, such as felt, which are neither woven nor knitted. Some non-woven materials lack sufficient strength unless densified or reinforced by a backing. In recent years, non-wovens have become an alternative to polyurethane foam.

A cloth diaper or a cloth nappy, also known as reusable diaper or reusable nappy, is a diaper made from textiles such as natural fibers, human-made materials, or a combination of both. Cloth diapers are in contrast to disposable diapers, made from synthetic fibers and plastics. They are often made from industrial cotton which may be bleached white or left the fiber's natural color. Other natural fiber cloth materials include wool, bamboo, and unbleached hemp. Human-made materials such as an internal absorbent layer of microfiber toweling or an external waterproof layer of polyurethane laminate (PUL) may be used. Polyester fabrics microfleece or suedecloth are often used inside cloth diapers as a "stay-dry" wicking liner because of the non-absorbent properties of those synthetic fibers.

A superabsorbent polymer (SAP) (also called slush powder) is a water-absorbing hydrophilic homopolymers or copolymers that can absorb and retain extremely large amounts of a liquid relative to its own mass.

A swim diaper or swim nappy is a diaper that is made for those who have fecal incontinence, which is worn underneath a bathing suit, or as a bathing suit. Swim diapers can be reusable and disposable. They are not intended to be absorbent, but only to contain solid waste (feces); the lack of absorbency prevents the swim diaper from swelling with water.
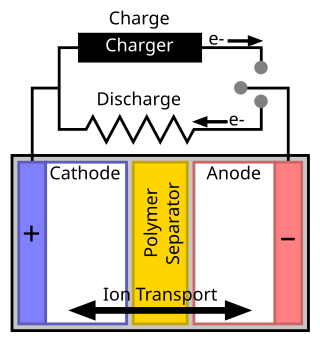
A separator is a permeable membrane placed between a battery's anode and cathode. The main function of a separator is to keep the two electrodes apart to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current in an electrochemical cell.

An alkaline anion-exchange membrane fuel cell (AAEMFC), also known as anion-exchange membrane fuel cells (AEMFCs), alkaline membrane fuel cells (AMFCs), hydroxide-exchange membrane fuel cells (HEMFCs), or solid alkaline fuel cells (SAFCs) is a type of alkaline fuel cell that uses an anion-exchange membrane to separate the anode and cathode compartments.

Kuzhikalail M. Abraham is an American scientist, a recognized expert on lithium-ion and lithium-ion polymer batteries and is the inventor of the ultrahigh energy density lithium–air battery. Abraham is the principal of E-KEM Sciences in Needham, Massachusetts and a professor at the Northeastern University Center for Renewable Energy Technologies, Northeastern University, in Boston, Massachusetts.
Dioxide Materials was founded in 2009 in Champaign, Illinois, and is now headquartered in Boca Raton, Florida. Its main business is to develop technology to lower the world's carbon footprint. Dioxide Materials is developing technology to convert carbon dioxide, water and renewable energy into carbon-neutral gasoline (petrol) or jet fuel. Applications include CO2 recycling, sustainable fuels production and reducing curtailment of renewable energy(i.e. renewable energy that could not be used by the grid).

Anion exchange membrane(AEM) electrolysis is the electrolysis of water that utilises a semipermeable membrane that conducts hydroxide ions (OH−) called an anion exchange membrane. Like a proton-exchange membrane (PEM), the membrane separates the products, provides electrical insulation between electrodes, and conducts ions. Unlike PEM, AEM conducts hydroxide ions. The major advantage of AEM water electrolysis is that a high-cost noble metal catalyst is not required, low-cost transition metal catalyst can be used instead. AEM electrolysis is similar to alkaline water electrolysis, which uses a non-ion-selective separator instead of an anion-exchange membrane.

















