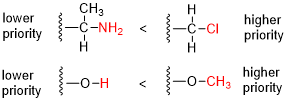Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4.
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles.

In organic chemistry, the cycloalkanes are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring, and all of the carbon-carbon bonds are single. The larger cycloalkanes, with more than 20 carbon atoms are typically called cycloparaffins. All cycloalkanes are isomers of alkenes.

In chemistry, an open-chain compound or acyclic compound is a compound with a linear structure, rather than a cyclic one. An open-chain compound having no side groups is called a straight-chain compound. Many of the simple molecules of organic chemistry, such as the alkanes and alkenes, have both linear and ring isomers, that is, both acyclic and cyclic. For those with 4 or more carbons, the linear forms can have straight-chain or branched-chain isomers. The lowercase prefix n- denotes the straight-chain isomer; for example, n-butane is straight-chain butane, whereas i-butane is isobutane. Cycloalkanes are isomers of alkenes, not of alkanes, because the ring's closure involves a C-C bond. Having no rings, all open-chain compounds are aliphatic.

In organic chemistry, an alicyclic compound contains one or more all-carbon rings which may be either saturated or unsaturated, but do not have aromatic character. Alicyclic compounds may have one or more aliphatic side chains attached.

Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds ('mancude'). They have the general formula CnHn (when n is an even number) or CnHn+1 (when n is an odd number). The IUPAC accepts the use of 'annulene nomenclature' in naming carbocyclic ring systems with 7 or more carbon atoms, using the name '[n]annulene' for the mancude hydrocarbon with n carbon atoms in its ring, though in certain contexts (e.g., discussions of aromaticity for different ring sizes), smaller rings (n = 3 to 6) can also be informally referred to as annulenes. Using this form of nomenclature 1,3,5,7-cyclooctatetraene is [8]annulene and benzene is [6]annulene (and occasionally referred to as just 'annulene').
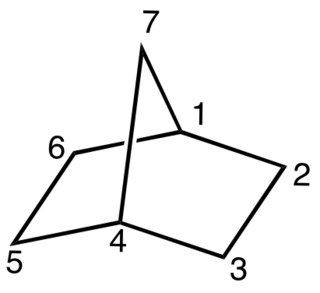
A bicyclic molecule is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic, or heterocyclic, like DABCO. Moreover, the two rings can both be aliphatic, or can be aromatic, or a combination of aliphatic and aromatic.
Class A metals are metals that form hard acids. Hard acids are acids with relatively ionic bonds. These metals, such as iron, aluminium, titanium, sodium, calcium, and the lanthanides, would rather bond with fluorine than iodine. They form stable products with hard bases, which are bases with ionic bonds. They target molecules such as phospholipids, nucleic acids, and ATP.

In organic chemistry, spiro compounds are compounds that have at least two molecular rings with only one common atom. The simplest spiro compounds are bicyclic, or have a bicyclic portion as part of the larger ring system, in either case with the two rings connected through the defining single common atom. The one common atom connecting the participating rings distinguishes spiro compounds from other bicyclics: from isolated ring compounds like biphenyl that have no connecting atoms, from fused ring compounds like decalin having two rings linked by two adjacent atoms, and from bridged ring compounds like norbornane with two rings linked by two non-adjacent atoms.

A cyclic compound is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon, none of the atoms are carbon, or where both carbon and non-carbon atoms are present. Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size numbers in the many billions.

In coordination chemistry, hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta'). For example, η2 describes a ligand that coordinates through 2 contiguous atoms. In general the η-notation only applies when multiple atoms are coordinated. In addition, if the ligand coordinates through multiple atoms that are not contiguous then this is considered denticity, and the κ-notation is used once again. When naming complexes care should be taken not to confuse η with μ ('mu'), which relates to bridging ligands.

In organic chemistry, annulation is a chemical reaction in which a new ring is constructed on a molecule.

In coordination chemistry, denticity refers to the number of donor groups in a given ligand that bind to the central metal atom in a coordination complex. In many cases, only one atom in the ligand binds to the metal, so the denticity equals one, and the ligand is said to be monodentate. Ligands with more than one bonded atom are called polydentate or multidentate. The denticity of a ligand is described with the Greek letter κ ('kappa'). For example, κ6-EDTA describes an EDTA ligand that coordinates through 6 non-contiguous atoms.

A chemical compound is a chemical substance composed of many identical molecules containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed.
In organic chemistry, Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system, is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no more than ten ring members. Some common heterocyclic compounds have retained names that do not follow the Hantzsch–Widman pattern.

In organic chemistry, a moiety is a part of a molecule that is given a name because it is identified as a part of other molecules as well.
In chemistry, a retained name is a name for a chemical compound, that is recommended for use by a system of chemical nomenclature, but that is not exactly systematic. Retained names are often used for the most fundamental parts of a nomenclature system: almost all the chemical elements have retained names rather than being named systematically, as do the first four alkanes, benzene and most simple heterocyclic compounds. Water and ammonia are other examples of retained names.
A ring forming reaction or ring-closing reaction in organic chemistry is a general term for a variety of reactions that introduce one or more rings into a molecule. A heterocycle forming reaction is such a reaction that introduces a new heterocycle. Important classes of ring forming reactions include annulations and cycloadditions.
In chemical nomenclature, a descriptor is a notational prefix placed before the systematic substance name, which describes the configuration or the stereochemistry of the molecule. Some listed descriptors are only of historical interest and should not be used in publications anymore as they do not correspond with the modern recommendations of the IUPAC. Stereodescriptors are often used in combination with locants to clearly identify a chemical structure unambiguously.