A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic.

Peritonitis is inflammation of the localized or generalized peritoneum, the lining of the inner wall of the abdomen and cover of the abdominal organs. Symptoms may include severe pain, swelling of the abdomen, fever, or weight loss. One part or the entire abdomen may be tender. Complications may include shock and acute respiratory distress syndrome.

The pleural cavity, or pleural space, is the potential space between the pleurae of the pleural sac that surrounds each lung. A small amount of serous pleural fluid is maintained in the pleural cavity to enable lubrication between the membranes, and also to create a pressure gradient.

Ascites is the abnormal build-up of fluid in the abdomen. Technically, it is more than 25 ml of fluid in the peritoneal cavity, although volumes greater than one liter may occur. Symptoms may include increased abdominal size, increased weight, abdominal discomfort, and shortness of breath. Complications can include spontaneous bacterial peritonitis.

Nephrotic syndrome is a collection of symptoms due to kidney damage. This includes protein in the urine, low blood albumin levels, high blood lipids, and significant swelling. Other symptoms may include weight gain, feeling tired, and foamy urine. Complications may include blood clots, infections, and high blood pressure.

A pleural effusion is accumulation of excessive fluid in the pleural space, the potential space that surrounds each lung. Under normal conditions, pleural fluid is secreted by the parietal pleural capillaries at a rate of 0.6 millilitre per kilogram weight per hour, and is cleared by lymphatic absorption leaving behind only 5–15 millilitres of fluid, which helps to maintain a functional vacuum between the parietal and visceral pleurae. Excess fluid within the pleural space can impair inspiration by upsetting the functional vacuum and hydrostatically increasing the resistance against lung expansion, resulting in a fully or partially collapsed lung.

An exudate is a fluid released by an organism through pores or a wound, a process known as exuding or exudation. Exudate is derived from exude 'to ooze' from Latin exsūdāre 'to sweat'.

Pleural empyema is a collection of pus in the pleural cavity caused by microorganisms, usually bacteria. Often it happens in the context of a pneumonia, injury, or chest surgery. It is one of the various kinds of pleural effusion. There are three stages: exudative, when there is an increase in pleural fluid with or without the presence of pus; fibrinopurulent, when fibrous septa form localized pus pockets; and the final organizing stage, when there is scarring of the pleura membranes with possible inability of the lung to expand. Simple pleural effusions occur in up to 40% of bacterial pneumonias. They are usually small and resolve with appropriate antibiotic therapy. If however an empyema develops additional intervention is required.

Carnivore protoparvovirus 1 is a species of parvovirus that infects carnivorans. It causes a highly contagious disease in both dogs and cats separately. The disease is generally divided into two major genogroups: FPV containing the classical feline panleukopenia virus (FPLV), and CPV-2 containing the canine parvovirus type 2 (CPV-2) which appeared in the 1970s.

Feline infectious peritonitis (FIP) is a common and aberrant immune response in cats to infection with feline coronavirus (FCoV).

A chylothorax is an abnormal accumulation of chyle, a type of lipid-rich lymph, in the pleural space surrounding the lung. The lymphatic vessels of the digestive system normally return lipids absorbed from the small bowel via the thoracic duct, which ascends behind the esophagus to drain into the left brachiocephalic vein. If normal thoracic duct drainage is disrupted, either due to obstruction or rupture, chyle can leak and accumulate within the negative-pressured pleural space. In people on a normal diet, this fluid collection can sometimes be identified by its turbid, milky white appearance, since chyle contains emulsified triglycerides.
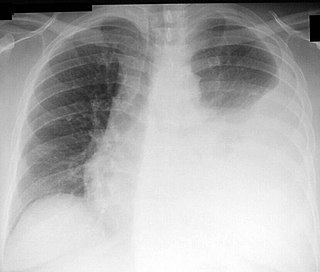
Thoracentesis, also known as thoracocentesis, pleural tap, needle thoracostomy, or needle decompression, is an invasive medical procedure to remove fluid or air from the pleural space for diagnostic or therapeutic purposes. A cannula, or hollow needle, is carefully introduced into the thorax, generally after administration of local anesthesia. The procedure was first performed by Morrill Wyman in 1850 and then described by Henry Ingersoll Bowditch in 1852.
Transudate is extravascular fluid with low protein content and a low specific gravity. It has low nucleated cell counts and the primary cell types are mononuclear cells: macrophages, lymphocytes and mesothelial cells. For instance, an ultrafiltrate of blood plasma is transudate. It results from increased fluid pressures or diminished colloid oncotic forces in the plasma.

A pericardial effusion is an abnormal accumulation of fluid in the pericardial cavity. The pericardium is a two-part membrane surrounding the heart: the outer fibrous connective membrane and an inner two-layered serous membrane. The two layers of the serous membrane enclose the pericardial cavity between them. This pericardial space contains a small amount of pericardial fluid, normally 15-50 mL in volume. The pericardium, specifically the pericardial fluid provides lubrication, maintains the anatomic position of the heart in the chest (levocardia), and also serves as a barrier to protect the heart from infection and inflammation in adjacent tissues and organs.
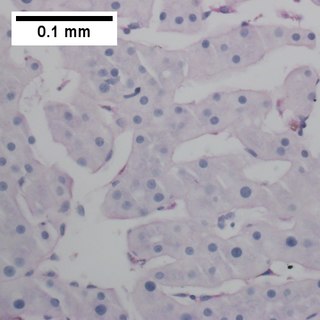
Primary effusion lymphoma (PEL) is classified as a diffuse large B cell lymphoma. It is a rare malignancy of plasmablastic cells that occurs in individuals that are infected with the Kaposi's sarcoma-associated herpesvirus. Plasmablasts are immature plasma cells, i.e. lymphocytes of the B-cell type that have differentiated into plasmablasts but because of their malignant nature do not differentiate into mature plasma cells but rather proliferate excessively and thereby cause life-threatening disease. In PEL, the proliferating plasmablastoid cells commonly accumulate within body cavities to produce effusions, primarily in the pleural, pericardial, or peritoneal cavities, without forming a contiguous tumor mass. In rare cases of these cavitary forms of PEL, the effusions develop in joints, the epidural space surrounding the brain and spinal cord, and underneath the capsule which forms around breast implants. Less frequently, individuals present with extracavitary primary effusion lymphomas, i.e., solid tumor masses not accompanied by effusions. The extracavitary tumors may develop in lymph nodes, bone, bone marrow, the gastrointestinal tract, skin, spleen, liver, lungs, central nervous system, testes, paranasal sinuses, muscle, and, rarely, inside the vasculature and sinuses of lymph nodes. As their disease progresses, however, individuals with the classical effusion-form of PEL may develop extracavitary tumors and individuals with extracavitary PEL may develop cavitary effusions.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.

Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of pyruvate to lactate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another.
Feline coronavirus (FCoV) is a positive-stranded RNA virus that infects cats worldwide. It is a coronavirus of the species Alphacoronavirus 1, which includes canine coronavirus (CCoV) and porcine transmissible gastroenteritis coronavirus (TGEV). FCoV has two different forms: feline enteric coronavirus (FECV), which infects the intestines, and feline infectious peritonitis virus (FIPV), which causes the disease feline infectious peritonitis (FIP).
Pandy's test is done on the CSF to detect the elevated levels of proteins. This test is named after the Hungarian neurologist, Pándy Kálmán (1868–1945) who developed this test in the year 1910.

GS-441524 is a nucleoside analogue antiviral drug which was developed by Gilead Sciences. It is the main plasma metabolite of the antiviral prodrug remdesivir, and has a half-life of around 24 hours in human patients. Remdesivir and GS-441524 were both found to be effective in vitro against feline coronavirus strains responsible for feline infectious peritonitis (FIP), a lethal systemic disease affecting domestic cats. Remdesivir was never tested in cats, but GS-441524 has been found to be effective treatment for FIP.















