
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils.
Essential fatty acids, or EFAs, are fatty acids that are required by humans and other animals for normal physiological function that cannot be synthesized in the body. As they are not synthesized in the body, the essential fatty acids – alpha-linolenic acid (ALA) and linoleic acid – must be obtained from food or from a dietary supplement. Essential fatty acids are needed for various cellular metabolic processes and for the maintenance and function of tissues and organs. These fatty acids also are precursors to vitamins, cofactors, and derivatives, including prostaglandins, leukotrienes, thromboxanes, lipoxins, and others.
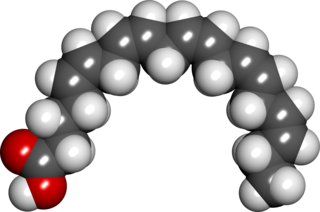
Eicosapentaenoic acid (EPA; also icosapentaenoic acid) is an omega−3 fatty acid. In physiological literature, it is given the name 20:5(n−3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.

Docosahexaenoic acid (DHA) is an omega−3 fatty acid that is an important component of the human brain, cerebral cortex, skin, and retina. It is given the fatty acid notation 22:6(n−3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk (breast milk), fatty fish, fish oil, or algae oil. The consumption of DHA (e.g., from fatty fish such as salmon, herring, mackerel and sardines) contributes to numerous physiological benefits, including cognition. As a component of neuronal membranes, the function of DHA is to support neuronal conduction and to allow the optimal functioning of neuronal membrane proteins (such as receptors and enzymes).

Bombykol is a pheromone released by the female silkworm moth to attract mates. It is also the sex pheromone in the wild silk moth. Discovered by Adolf Butenandt in 1959, it was the first pheromone to be characterized chemically.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linoleic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
Eicosatetraenoic acid (ETA) designates any straight chain tetra-unsaturated 20-carbon fatty acid. These compound are classified as polyunsaturated fatty acids (PUFA). The pure compounds, which are encountered rarely, are colorless oils. Two isomers, both of them essential fatty acids, are of particular interest:
Mycolic acids are long fatty acids found in the cell walls of Mycobacteriales taxon, a group of bacteria that includes Mycobacterium tuberculosis, the causative agent of the disease tuberculosis. They form the major component of the cell wall of many Mycobacteriales species. Despite their name, mycolic acids have no biological link to fungi; the name arises from the filamentous appearance their presence gives Mycobacteriales under high magnification. The presence of mycolic acids in the cell wall also gives Mycobacteriales a distinct gross morphological trait known as "cording". Mycolic acids were first isolated by Stodola et al. in 1938 from an extract of M. tuberculosis.
There is a wide variety of fatty acids found in nature. Two classes of fatty acids are considered essential, the omega-3 and omega-6 fatty acids. Essential fatty acids are necessary for humans but cannot be synthesized by the body and must therefore be obtained from food. Omega-3 and omega-6 are used in some cellular signaling pathways and are involved in mediating inflammation, protein synthesis, and metabolic pathways in the human body.
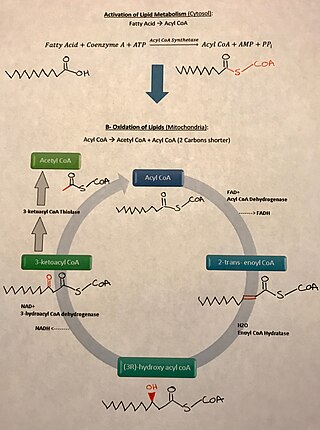
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells. In addition to cytosolic fatty acid synthesis, there is also mitochondrial fatty acid synthesis (mtFASII), in which malonyl-CoA is formed from malonic acid with the help of malonyl-CoA synthetase (ACSF3), which then becomes the final product octanoyl-ACP (C8) via further intermediate steps.

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.

In enzymology, an acyl-[acyl-carrier-protein] desaturase (EC 1.14.19.2) is an enzyme that catalyzes the chemical reaction

Linoleoyl-CoA desaturase (also Delta 6 desaturase, EC 1.14.19.3) is an enzyme that converts between types of fatty acids, which are essential nutrients in the human body. The enzyme mainly catalyzes the chemical reaction

Stearoyl-CoA desaturase is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the formation of monounsaturated fatty acids (MUFAs), specifically oleate and palmitoleate from stearoyl-CoA and palmitoyl-CoA. Oleate and palmitoleate are major components of membrane phospholipids, cholesterol esters and alkyl-diacylglycerol. In humans, the enzyme is present in two isoforms, encoded respectively by the SCD1 and SCD5 genes.

Fatty acid desaturase 2 (FADS2) is an enzyme that in humans is encoded by the FADS2 gene.

Fatty acid desaturase 1 (FADS1) is an enzyme that in humans is encoded by the FADS1 gene.
Sapienic acid is a fatty acid that is a major component of human sebum. Unique to humans, it takes its scientific name from the root sapiens. The equivalent fatty acid in mouse sebum is palmitoleic acid. Sapienic acid salts, esters, anion, and conjugate base are known as sapienates.
Delta12-fatty-acid desaturase (EC 1.14.19.6, Delta12 fatty acid desaturase, Delta12(omega6)-desaturase, oleoyl-CoA Delta12 desaturase, Delta12 desaturase, Delta12-desaturase) is an enzyme with systematic name acyl-CoA,hydrogen donor:oxygen Delta12-oxidoreductase. This enzyme catalyses the following chemical reaction

Sciadonic acid, also known as eicosatrienoic acid, is a polyunsaturated fatty acid. In regard to its structure, 5Z,11Z,14Z-eicosa-5,11,14-trienoic acid has 3 double bonds in the 5, 11, and 14 positions all of which are in the cis configuration. It is further classified as Δ5-fatty, and an omega-6 acid due to the methylene interrupted double bond at carbon-5 and a final double bond 6 carbons away from the methylene tail of the hydrocarbon. Sciadonic acid is a naturally occurring compound and has been found to play a role as a plant metabolite, commonly found in pine nut oil. Furthermore, there have been propositions of several health applications for sciadonic acid as an anti-inflammatory agent. Sharing close structural similarity to arachidonic acid, sciadonic acid acts as a replacement phospholipid in the corresponding biochemical pathways.











