Related Research Articles

Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Biophysical research shares significant overlap with biochemistry, molecular biology, physical chemistry, physiology, nanotechnology, bioengineering, computational biology, biomechanics, developmental biology and systems biology.

Peripheral membrane proteins, or extrinsic membrane proteins, are membrane proteins that adhere only temporarily to the biological membrane with which they are associated. These proteins attach to integral membrane proteins, or penetrate the peripheral regions of the lipid bilayer. The regulatory protein subunits of many ion channels and transmembrane receptors, for example, may be defined as peripheral membrane proteins. In contrast to integral membrane proteins, peripheral membrane proteins tend to collect in the water-soluble component, or fraction, of all the proteins extracted during a protein purification procedure. Proteins with GPI anchors are an exception to this rule and can have purification properties similar to those of integral membrane proteins.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.
Implicit solvation is a method to represent solvent as a continuous medium instead of individual “explicit” solvent molecules, most often used in molecular dynamics simulations and in other applications of molecular mechanics. The method is often applied to estimate free energy of solute-solvent interactions in structural and chemical processes, such as folding or conformational transitions of proteins, DNA, RNA, and polysaccharides, association of biological macromolecules with ligands, or transport of drugs across biological membranes.
David S. Cafiso is an American biochemist and a Professor of Chemistry at the University of Virginia. His research focuses on membrane proteins and cell signaling, and is primarily supported by grants from the National Institute of Health.
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion of proteins and other bio-molecules within the membrane, there-by affecting the functions of these things.
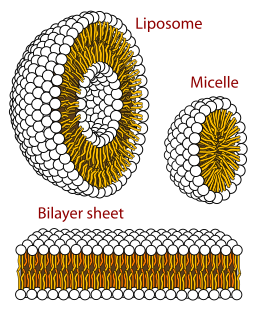
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.

Myristoylated alanine-rich C-kinase substrate is a protein that in humans is encoded by the MARCKS gene. It plays important roles in cell shape, cell motility, secretion, transmembrane transport, regulation of the cell cycle, and neural development. Recently, MARCKS has been implicated in the exocytosis of a number of vesicles and granules such as mucin and chromaffin. It is also the name of a protein family, of which MARCKS is the most studied member. They are intrinsically disordered proteins, with an acidic pH, with high proportions of alanine, glycine, proline, and glutamic acid. They are membrane-bound through a lipid anchor at the N-terminus, and a polybasic domain in the middle. They are regulated by Ca2+/calmodulin and protein kinase C. In their unphosphorylated form, they bind to actin filaments, causing them to crosslink, and sequester acidic membrane phospholipids such as PIP2.
Lipid microdomains are formed when lipids undergo lateral phase separations yielding stable coexisting lamellar domains. These phase separations can be induced by changes in temperature, pressure, ionic strength or by the addition of divalent cations or proteins. The question of whether such lipid microdomains observed in model lipid systems also exist in biomembranes had motivated considerable research efforts. Lipid domains are not readily isolated and examined as unique species, in contrast to the examples of lateral heterogeneity. One can disrupt the membrane and demonstrate a heterogeneous range of composition in the population of the resulting vesicles or fragments. Electron microscopy can also be used to demonstrate lateral inhomogeneities in biomembranes.
Protein–lipid interaction is the influence of membrane proteins on the lipid physical state or vice versa.
One property of a lipid bilayer is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a transition (melt) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk, migrate over long distances.

In membrane biology, fusion is the process by which two initially distinct lipid bilayers merge their hydrophobic cores, resulting in one interconnected structure. If this fusion proceeds completely through both leaflets of both bilayers, an aqueous bridge is formed and the internal contents of the two structures can mix. Alternatively, if only one leaflet from each bilayer is involved in the fusion process, the bilayers are said to be hemifused. In hemifusion, the lipid constituents of the outer leaflet of the two bilayers can mix, but the inner leaflets remain distinct. The aqueous contents enclosed by each bilayer also remain separated.
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in a simplified and well-controlled environment, and increasingly in bottom-up synthetic biology for the construction of artificial cells. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.

The presence of ethanol can lead to the formations of non-lamellar phases also known as non-bilayer phases. Ethanol has been recognized as being an excellent solvent in an aqueous solution for inducing non-lamellar phases in phospholipids. The formation of non-lamellar phases in phospholipids is not completely understood, but it is significant that this amphiphilic molecule is capable of doing so. The formation of non-lamellar phases is significant in biomedical studies which include drug delivery, the transport of polar and non-polar ions using solvents capable of penetrating the biomembrane, increasing the elasticity of the biomembrane when it is being disrupted by unwanted substances and functioning as a channel or transporter of biomaterial.
The Deep Carbon Observatory (DCO) is a global research program designed to transform understanding of carbon's role in Earth. DCO is a community of scientists, including biologists, physicists, geoscientists and chemists, whose work crosses several traditional disciplinary lines to develop the new, integrative field of deep carbon science. To complement this research, the DCO's infrastructure includes public engagement and education, online and offline community support, innovative data management, and novel instrumentation development.
Stephen H. White is an American Biophysicist, academic, and author. He is a Professor Emeritus of Physiology and Biophysics at the University of California, Irvine.

Anthony Watts is a British biochemist and Professor of Biochemistry at the University of Oxford and C W Maplethorpe Fellow in Biological Sciences and tutor at St. Hugh's College, Oxford. He is a fellow of the Royal Chemical Society, the Institute of Physics, Royal Society of Biology and Biophysical Society. He was managing director of the European Biophysics Journal, and is a co-opted member of the European Biophysical Societies' Association (EBSA), chair of the British Biophysical Society and chair of the Scientific Committee for the IUPAB/EBSA/BBS/IoP Biophysics congress, 2017. He was President of EBSA (2017-2019) and elected President-elect of IUPAB in 2021.
Craig E. Manning is a professor of geology and geochemistry in the Department of Earth, Planetary, and Space Sciences at the University of California, Los Angeles, where he served as department chair between 2009 and 2012. Manning's research interests include water chemistry, thermodynamics, gas chemistry, geochemistry, igneous petrology, and metamorphic petrology.
Ka Yee Christina Lee is a Professor of Chemistry and the Provost at the University of Chicago, where she succeeded Daniel Diermeier on February 1, 2020. She works on membrane biophysics, including protein–lipid interactions, Alzheimer's disease and respiratory distress syndrome. She is a Fellow of the American Institute for Medical and Biological Engineering and American Physical Society.
Suzanne Frances Scarlata is the Richard Whitcomb Professor at Worcester Polytechnic Institute. She is known for her work on how cells respond to hormones and neurotransmitters. She is an elected fellow of the American Association for the Advancement of Science.
References
- 1 2 3 4 "Curriculum Vitae". www.ccb.tu-dortmund.de. Retrieved 2017-07-19.
- ↑ "Executive Board". RESOLV. 2017-04-19. Retrieved 2017-07-19.
- ↑ "DCO Scientific Steering Committees | Deep Carbon Observatory Portal". deepcarbon.net. Archived from the original on 2017-08-02. Retrieved 2017-07-19.
- ↑ Winter, Roland (2002-03-25). "Synchrotron X-ray and neutron small-angle scattering of lyotropic lipid mesophases, model biomembranes and proteins in solution at high pressure". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1595 (1): 160–184. doi:10.1016/S0167-4838(01)00342-9. PMID 11983394.
- ↑ Kapoor, Shobhna; Werkmüller, Alexander; Goody, Roger S.; Waldmann, Herbert; Winter, Roland (2013-04-24). "Pressure Modulation of Ras–Membrane Interactions and Intervesicle Transfer". Journal of the American Chemical Society. 135 (16): 6149–6156. doi:10.1021/ja312671j. ISSN 0002-7863. PMID 23560466.