
3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy, and molly, is an empathogen–entactogenic drug with stimulant and minor psychedelic properties. In studies, it has been used alongside psychotherapy in the treatment of post-traumatic stress disorder (PTSD) and social anxiety in autism spectrum disorder. The purported pharmacological effects that may be prosocial include altered sensations, increased energy, empathy, and pleasure. When taken by mouth, effects begin in 30 to 45 minutes and last three to six hours.

Empathogens or entactogens are a class of psychoactive drugs that induce the production of experiences of emotional communion, oneness, relatedness, emotional openness—that is, empathy or sympathy—as particularly observed and reported for experiences with 3,4-methylenedioxymethamphetamine (MDMA). This class of drug is distinguished from the classes of hallucinogen or psychedelic, and amphetamine or stimulants. Major members of this class include MDMA, MDA, MDEA, MDOH, MBDB, 5-APB, 5-MAPB, 6-APB, 6-MAPB, methylone, mephedrone, GHB, αMT, and αET, MDAI among others. Most entactogens are phenethylamines and amphetamines, although several, such as αMT and αET, are tryptamines. When referring to MDMA and its counterparts, the term MDxx is often used. Entactogens are sometimes incorrectly referred to as hallucinogens or stimulants, although many entactogens such as ecstasy exhibit psychedelic or stimulant properties as well.

3,4-Methylenedioxyamphetamine (MDA), sometimes referred to as “sass,” is an empathogen-entactogen, stimulant, and psychedelic drug of the amphetamine family that is encountered mainly as a recreational drug. In its pharmacology, MDA is a serotonin–norepinephrine–dopamine releasing agent (SNDRA). In most countries, the drug is a controlled substance and its possession and sale are illegal.
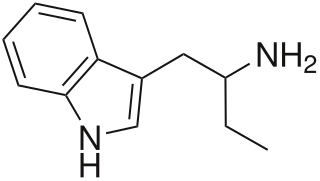
α-Ethyltryptamine, also known as etryptamine, is an entactogen and stimulant drug of the tryptamine family. It was originally developed and marketed as an antidepressant under the brand name Monase by Upjohn in the 1960s before being withdrawn due to toxicity.

para-Methoxyamphetamine (PMA), also known as 4-methoxyamphetamine (4-MA), is a designer drug of the amphetamine class with serotonergic effects. Unlike other similar drugs of this family, PMA does not produce stimulant, euphoriant, or entactogen effects, and behaves more like an antidepressant in comparison, though it does have some psychedelic properties.

The norepinephrine transporter (NET), also known as noradrenaline transporter (NAT), is a protein that in humans is encoded by the solute carrier family 6 member 2 (SLC6A2) gene.
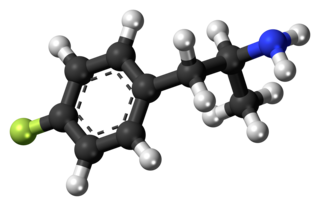
4-Fluoroamphetamine, also known as para-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine.

4-Methylthioamphetamine (4-MTA) is a designer drug of the substituted amphetamine class developed in the 1990s by a team led by David E. Nichols, an American pharmacologist and medical chemist, at Purdue University. It acts as a non-neurotoxic highly selective serotonin releasing agent (SSRA) in animals. 4-MTA is the methylthio derivative of amphetamine.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a substituted amphetamine and monoamine releaser similar to MDMA, but with substantially higher activity as a monoaminergic neurotoxin, thought to be due to the unrestrained release of both serotonin and dopamine by a metabolite. It is used as a neurotoxin by neurobiologists to selectively kill serotonergic neurons for research purposes, in the same way that 6-hydroxydopamine is used to kill dopaminergic neurons.

MDAI (5,6-methylenedioxy-2-aminoindane) is a drug developed in the 1990s by a team led by David E. Nichols at Purdue University. It acts as a non-neurotoxic and highly selective serotonin releasing agent (SSRA) in vitro and produces entactogen effects in humans.

A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the release of a monoamine neurotransmitter from the presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds.
A serenic, or antiaggressive agent, is a type of drug which reduces the capacity for irritability and aggression.

Difluoromethylenedioxyamphetamine is a substituted derivative of 3,4-methylenedioxyamphetamine (MDA), which was developed by Daniel Trachsel and coworkers, along with the corresponding fluorinated derivatives of MDMA, MDEA, BDB and MBDB, with the aim of finding a non-neurotoxic drug able to be used as a less harmful substitute for entactogenic drugs such as MDMA. Since a major route of the normal metabolism of these compounds is scission of the methylenedioxy ring, producing neurotoxic metabolites such as alpha-methyldopamine, it was hoped that the difluoromethylenedioxy bioisostere would show increased metabolic stability and less toxicity.

UWA-101 is a phenethylamine derivative researched as a potential treatment for Parkinson's disease. Its chemical structure is very similar to that of the illegal drug MDMA, the only difference being the replacement of the α-methyl group with an α-cyclopropyl group. MDMA has been found in animal studies and reported in unauthorised human self-experiments to be effective in the short-term relief of side-effects of Parkinson's disease therapy, most notably levodopa-induced dyskinesia. However the illegal status of MDMA and concerns about its potential for recreational use, neurotoxicity and potentially dangerous side effects mean that it is unlikely to be investigated for medical use in this application, and so alternative analogues were investigated.

para-Chloromethamphetamine is a stimulant that is the N-methyl derivative and prodrug of the neurotoxic drug para-chloroamphetamine (4-CA). It has been found to decrease serotonin in rats. Further investigation into the long-term effects of chloroamphetamines discovered that administration of 4-CMA caused a prolonged reduction in the levels of serotonin and the activity of tryptophan hydroxylase in the brain one month after injection of a single dose of the drug.
Una D. McCann is a board certified psychiatrist and researcher at Johns Hopkins School of Medicine in the Department of Psychiatry. She is also the Director of the Anxiety Disorders Program, and Co-Director of the Center for Interdisciplinary Sleep Medicine and Research, and Associate Program Director at the Johns Hopkins Bayview Medical Center. McCann is considered to be an expert in anxiety and stress disorders and her primary areas research revolves around amphetamine-induced monoamine neurotoxicity and neurobiology of anxiety disorders.

A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine.

ODMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzoxadiazole ring. TDMA and SeDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.

TDMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzothiadiazole ring. ODMA and SeDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.

SeDMA is a bioisosteric analogue of 3,4-methylenedioxy-N-methylamphetamine (MDMA) which was developed in an attempt to create an improved MDMA alternative for potential clinical use. It is the analogue of MDMA in which the 1,3-benzodioxole ring has been replaced with a 2,1,3-benzoselenadiazole ring. ODMA and TDMA are closely related analogues. ODMA, TDMA, and SeDMA are releasing agents of serotonin, norepinephrine, and dopamine similarly to MDMA. However, they are less potent and efficacious in activating the serotonin 5-HT2A, 5-HT2B, and 5-HT2C receptors than MDMA and show differing and potentially improved metabolic and pharmacokinetic properties in comparison. ODMA, TDMA, and SeDMA were first described in the scientific literature in June 2024.



















