Related Research Articles

Brownian motion, or pedesis, is the random motion of particles suspended in a medium.
Intermolecular forces (IMF) are the forces which mediate interaction between atoms, including forces of attraction or repulsion which act between atoms and other types of neighboring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics.
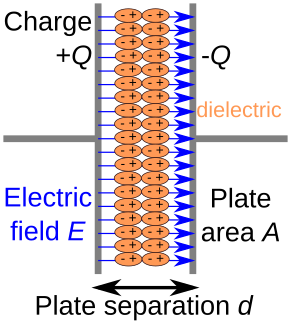
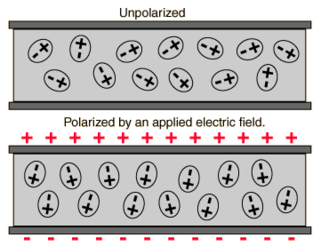
A dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor but only slightly shift from their average equilibrium positions causing dielectric polarization. Because of dielectric polarization, positive charges are displaced in the direction of the field and negative charges shift in the direction opposite to the field. This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly bonded molecules, those molecules not only become polarized, but also reorient so that their symmetry axes align to the field.
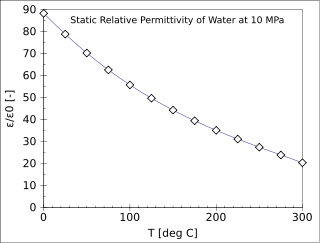
The relative permittivity, or dielectric constant, of a material is its (absolute) permittivity expressed as a ratio relative to the vacuum permittivity.
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ε (epsilon), is a measure of the electric polarizability of a dielectric. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the material. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor.

Electrophoresis is the motion of dispersed particles relative to a fluid under the influence of a spatially uniform electric field. Electrophoresis of positively charged particles (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles (anions) is sometimes called anaphoresis.

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy where rotational, vibrational and electronic energy changes occur simultaneously.
In electricity (electromagnetism), the electric susceptibility is a dimensionless proportionality constant that indicates the degree of polarization of a dielectric material in response to an applied electric field. The greater the electric susceptibility, the greater the ability of a material to polarize in response to the field, and thereby reduce the total electric field inside the material(and store energy). It is in this way that the electric susceptibility influences the electric permittivity of the material and thus influences many other phenomena in that medium, from the capacitance of capacitors to the speed of light.
In physics, the electric displacement field or electric induction is a vector field that appears in Maxwell's equations. It accounts for the effects of free and bound charge within materials. "D" stands for "displacement", as in the related concept of displacement current in dielectrics. In free space, the electric displacement field is equivalent to flux density, a concept that lends understanding to Gauss's law. In the International System of Units (SI), it is expressed in units of coulomb per meter square (C⋅m−2).
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, inasmuch as matter is made up of elementary particles which have an electric charge, namely protons and electrons. When subject to an electric field, the negatively charged electrons and positively charged atomic nuclei are subject to opposite forces and undergo charge separation. Polarizability is responsible for a material's dielectric constant and, at high (optical) frequencies, its refractive index.

According to quantum mechanics, atoms and molecules can only hold certain defined quantities of energy, or exist in specific states. When such quanta of electromagnetic radiation are emitted or absorbed by an atom or molecule, energy of the radiation changes the state of the atom or molecule from an initial state to a final state. An absorption band is a range of wavelengths, frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance.

Dielectric spectroscopy measures the dielectric properties of a medium as a function of frequency. It is based on the interaction of an external field with the electric dipole moment of the sample, often expressed by permittivity.

Dielectric heating, also known as electronic heating, radio frequency heating, and high-frequency heating, is the process in which a radio frequency (RF) alternating electric field, or radio wave or microwave electromagnetic radiation heats a dielectric material. At higher frequencies, this heating is caused by molecular dipole rotation within the dielectric.
Dielectric loss quantifies a dielectric material's inherent dissipation of electromagnetic energy. It can be parameterized in terms of either the loss angleδ or the corresponding loss tangent tan δ. Both refer to the phasor in the complex plane whose real and imaginary parts are the resistive (lossy) component of an electromagnetic field and its reactive (lossless) counterpart.
Electroacoustic phenomena arise when ultrasound propagates through a fluid containing ions. The associated particle motion generates electric signals because ions have electric charge. This coupling between ultrasound and electric field is called electroacoustic phenomena. The fluid might be a simple Newtonian liquid, or complex heterogeneous dispersion, emulsion or even a porous body. There are several different electroacoustic effects depending on the nature of the fluid.
In dielectric spectroscopy, large frequency dependent contributions to the dielectric response, especially at low frequencies, may come from build-ups of charge. This Maxwell–Wagner–Sillars polarization, occurs either at inner dielectric boundary layers on a mesoscopic scale, or at the external electrode-sample interface on a macroscopic scale. In both cases this leads to a separation of charges. The charges are often separated over a considerable distance, and the contribution to dielectric loss can therefore be orders of magnitude larger than the dielectric response due to molecular fluctuations.
Brendan Kevin Patrick Scaife FTCD, MRIA, Boyle Laureate, is an Irish academic engineer and physicist who carried out pioneering work on the theory of dielectrics. Scaife founded the Dielectrics Group in Trinity College Dublin where he is Fellow Emeritus and formerly Professor of Electromagnetism, and previously to that a professor of engineering science. Scaife showed that in a linear system the decay function is directly proportional to the autocorrelation function of the corresponding fluctuating macroscopic variable, and proved how the spectral density of the dipole moment fluctuations of a dielectric body could be calculated from the frequency dependence of the complex permittivity, ε(ω) = ε'(ω) – iε"(ω). It was independent of Ryogo Kubo who in 1957 developed the corresponding theory for magnetic materials. The work was published prior to the work of Robert Cole in 1965 which is often cited.
Plasmonic nanoparticles are particles whose electron density can couple with electromagnetic radiation of wavelengths that are far larger than the particle due to the nature of the dielectric-metal interface between the medium and the particles: unlike in a pure metal where there is a maximum limit on what size wavelength can be effectively coupled based on the material size.
Dielectric absorption is the name given to the effect by which a capacitor, that has been charged for a long time, discharges only incompletely when briefly discharged. Although an ideal capacitor would remain at zero volts after being discharged, real capacitors will develop a small voltage from time-delayed dipole discharging, a phenomenon that is also called dielectric relaxation, "soakage", or "battery action". For some dielectrics, such as many polymer films, the resulting voltage may be less than 1–2% of the original voltage, but it can be as much as 15% for electrolytic capacitors. The voltage at the terminals generated by the dielectric absorption may possibly cause problems in the function of an electronic circuit or can be a safety risk to personnel. In order to prevent shocks, most very large capacitors are shipped with shorting wires that need to be removed before they are used and/or permanently connected bleeder resistors. When disconnected at one or both ends, DC high-voltage cables can also "recharge themselves" to dangerous voltages.
Radio-frequency welding, also known as dielectric welding and high-frequency welding, is a plastic welding process that utilizes high-frequency electric fields to induce heating and melting of thermoplastic base materials. The electric field is applied by a pair of electrodes after the parts being joined are clamped together. The clamping force is maintained until the joint solidifies. Advantages of this process are fast cycle times, automation, repeatability, and good weld appearance. Only plastics which have dipoles can be heated using radio waves and therefore not all plastics are able to be welded using this process. Also, this process is not well suited for thick or overly complex joints. The most common use of this process is lap joints or seals on thin plastic sheets or parts.
References
- ↑ Debye, P., Berichte der deutschen Physikalischen Gesellschaft, 15, 777 (1913)