
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.

Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils.

Abetalipoproteinemia is a disorder characterized by abnormal absorption of fat and fat-soluble vitamins from food. It is caused by a mutation in microsomal triglyceride transfer protein resulting in deficiencies in the apolipoproteins B-48 and B-100, which are used in the synthesis and exportation of chylomicrons and VLDL respectively. It is not to be confused with familial dysbetalipoproteinemia.
Essential fatty acids, or EFAs, are fatty acids that are required by humans and other animals for normal physiological function that cannot be synthesized in the body. As they are not synthesized in the body, the essential fatty acids – alpha-linolenic acid (ALA) and linoleic acid – must be obtained from food or from a dietary supplement. Essential fatty acids are needed for various cellular metabolic processes and for the maintenance and function of tissues and organs. These fatty acids also are precursors to vitamins, cofactors, and derivatives, including prostaglandins, leukotrienes, thromboxanes, lipoxins, and others.
A saturated fat is a type of fat in which the fatty acid chains have all single bonds between the carbon atoms. A fat known as a glyceride is made of two kinds of smaller molecules: a short glycerol backbone and fatty acids that each contain a long linear or branched chain of carbon (C) atoms. Along the chain, some carbon atoms are linked by single bonds (-C-C-) and others are linked by double bonds (-C=C-). A double bond along the carbon chain can react with a pair of hydrogen atoms to change into a single -C-C- bond, with each H atom now bonded to one of the two C atoms. Glyceride fats without any carbon chain double bonds are called saturated because they are "saturated with" hydrogen atoms, having no double bonds available to react with more hydrogen.

Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C (156.9 °F) °C and a pKa of 4.50.

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D ratio is 16:0. It is a major component of palm oil from the fruit of Elaeis guineensis, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats.
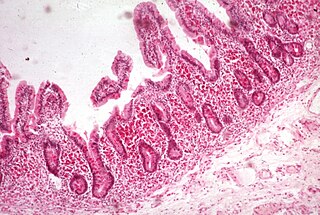
Malabsorption is a state arising from abnormality in absorption of food nutrients across the gastrointestinal (GI) tract. Impairment can be of single or multiple nutrients depending on the abnormality. This may lead to malnutrition and a variety of anaemias.
Butterfat or milkfat is the fatty portion of milk. Milk and cream are often sold according to the amount of butterfat they contain.

Retinyl palmitate, or vitamin A palmitate, is the ester of retinol (vitamin A) and palmitic acid, with formula C36H60O2. It is the most abundant form of vitamin A storage in animals.

Palmitoleic acid, or (9Z)-hexadec-9-enoic acid, is an omega-7 monounsaturated fatty acid (16:1n-7) with the formula CH3(CH2)5CH=CH(CH2)7COOH. It is a rare component of fats. It is a common constituent of the glycerides of human adipose tissue. It is present in all tissues but, in general, found in higher concentrations in the liver.

Monoglycerides are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.

A medium-chain triglyceride (MCT) is a triglyceride with two or three fatty acids having an aliphatic tail of 6–12 carbon atoms, i.e. a medium-chain fatty acid (MCFA). Rich food sources for commercial extraction of MCTs include palm kernel oil and coconut oil.
Lipid metabolism is the synthesis and degradation of lipids in cells, involving the breakdown and storage of fats for energy and the synthesis of structural and functional lipids, such as those involved in the construction of cell membranes. In animals, these fats are obtained from food and are synthesized by the liver. Lipogenesis is the process of synthesizing these fats. The majority of lipids found in the human body from ingesting food are triglycerides and cholesterol. Other types of lipids found in the body are fatty acids and membrane lipids. Lipid metabolism is often considered the digestion and absorption process of dietary fat; however, there are two sources of fats that organisms can use to obtain energy: from consumed dietary fats and from stored fat. Vertebrates use both sources of fat to produce energy for organs such as the heart to function. Since lipids are hydrophobic molecules, they need to be solubilized before their metabolism can begin. Lipid metabolism often begins with hydrolysis, which occurs with the help of various enzymes in the digestive system. Lipid metabolism also occurs in plants, though the processes differ in some ways when compared to animals. The second step after the hydrolysis is the absorption of the fatty acids into the epithelial cells of the intestinal wall. In the epithelial cells, fatty acids are packaged and transported to the rest of the body.
Vaccenic acid is a naturally occurring trans fatty acid and an omega-7 fatty acid. It is the predominant kind of trans-fatty acid found in human milk, in the fat of ruminants, and in dairy products such as milk, butter, and yogurt. Trans fat in human milk may depend on trans fat content in food. Vaccenic acid was discovered in 1928 in animal fats and butter. Mammals convert it into rumenic acid, a conjugated linoleic acid, where it shows anticarcinogenic properties. Cows milk had highest trans-vaccenic acid content in the first few days of the cows being milked, indicating that it is stockpiled similarly to omega-3 fatty acids.
Diacylglycerol oil is a cooking oil in which the ratio of triglycerides, also known as Triacylglycerols (TAGs), to diacylglycerols (DAGs) is shifted to contain mostly DAG, unlike conventional cooking oils, which are rich in TAGs. Vegetable DAG oil, for example, contains 80% DAG and is used as a 1:1 replacement for liquid vegetable oils in all applications.
The chronic endothelial injury hypothesis is one of two major mechanisms postulated to explain the underlying cause of atherosclerosis and coronary heart disease (CHD), the other being the lipid hypothesis. Although an ongoing debate involving connection between dietary lipids and CHD sometimes portrays the two hypotheses as being opposed, they are in no way mutually exclusive. Moreover, since the discovery of the role of LDL cholesterol (LDL-C) in the pathogenesis of atherosclerosis, the two hypotheses have become tightly linked by a number of molecular and cellular processes.

Milk fat globule membrane (MFGM) is a complex and unique structure composed primarily of lipids and proteins that surrounds milk fat globule secreted from the milk producing cells of humans and other mammals. It is a source of multiple bioactive compounds, including phospholipids, glycolipids, glycoproteins, and carbohydrates that have important functional roles within the brain and gut.












