
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver and are a major component of human skin oils.
Transesterification is the process of exchanging the organic functional group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile. Bases catalyze the reaction by removing a proton from the alcohol, thus making it more nucleophilic. The reaction can also be accomplished with the help of enzymes, particularly lipases.

Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and free fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include leptin, glucagon, epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.
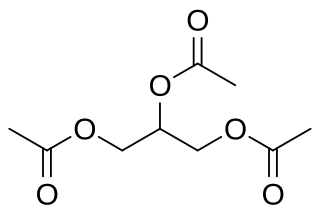
Glycerides, also known as acylglycerols, are esters formed from glycerol and fatty acids, and are generally very hydrophobic.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This process renders a product (chemistry) and by-products.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.
Lipid metabolism is the synthesis and degradation of lipids in cells, involving the breakdown and storage of fats for energy and the synthesis of structural and functional lipids, such as those involved in the construction of cell membranes. In animals, these fats are obtained from food and are synthesized by the liver. Lipogenesis is the process of synthesizing these fats. The majority of lipids found in the human body from ingesting food are triglycerides and cholesterol. Other types of lipids found in the body are fatty acids and membrane lipids. Lipid metabolism is often considered the digestion and absorption process of dietary fat; however, there are two sources of fats that organisms can use to obtain energy: from consumed dietary fats and from stored fat. Vertebrates use both sources of fat to produce energy for organs such as the heart to function. Since lipids are hydrophobic molecules, they need to be solubilized before their metabolism can begin. Lipid metabolism often begins with hydrolysis, which occurs with the help of various enzymes in the digestive system. Lipid metabolism also occurs in plants, though the processes differ in some ways when compared to animals. The second step after the hydrolysis is the absorption of the fatty acids into the epithelial cells of the intestinal wall. In the epithelial cells, fatty acids are packaged and transported to the rest of the body.

Monoacylglycerol lipase is an enzyme that, in humans, is encoded by the MGLL gene. MAGL is a 33-kDa, membrane-associated member of the serine hydrolase superfamily and contains the classical GXSXG consensus sequence common to most serine hydrolases. The catalytic triad has been identified as Ser122, His269, and Asp239.

Hormone-sensitive lipase (EC 3.1.1.79, HSL), also previously known as cholesteryl ester hydrolase (CEH), sometimes referred to as triacylglycerol lipase, is an enzyme that, in humans, is encoded by the LIPE gene, and catalyzes the following reaction:

Fatty acid esters (FAEs) are a type of ester that result from the combination of a fatty acid with an alcohol. When the alcohol component is glycerol, the fatty acid esters produced can be monoglycerides, diglycerides, or triglycerides. Dietary fats are chemically triglycerides.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×106 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.

In organic chemistry glycerolysis refers to any process in which chemical bonds are broken via a reaction with glycerol. The term refers almost exclusively to the transesterification reaction of glycerol with triglycerides (fats/oils) to form mixtures of monoglycerides and diglycerides. These find a variety of uses; as food emulsifiers, 'low fat' cooking oils and surfactants.
Glycerol monostearate, commonly known as GMS, is a monoglyceride commonly used as an emulsifier in foods. It takes the form of a white, odorless, and sweet-tasting flaky powder that is hygroscopic. Chemically it is the glycerol ester of stearic acid. It is also used as hydration powder in exercise formulas.

In biochemistry, lipase refers to a class of enzymes that catalyzes the hydrolysis of fats. Some lipases display broad substrate scope including esters of cholesterol, phospholipids, and of lipid-soluble vitamins and sphingomyelinases; however, these are usually treated separately from "conventional" lipases. Unlike esterases, which function in water, lipases "are activated only when adsorbed to an oil–water interface". Lipases perform essential roles in digestion, transport and processing of dietary lipids in most, if not all, organisms.

Mono- and diglycerides of fatty acids (E471) are a naturally occurring class of food additive composed of diglycerides and monoglycerides used as an emulsifier in foods such as infant formula, fresh pasta, jams and jellies, chocolate, creams, baked goods, and more. It is also used as a fruit coating agent. This mixture is also sometimes referred to as partial glycerides.
In the food industry and biochemistry, interesterification (IE) is a process that rearranges the fatty acids of a fat product, typically a mixture of triglycerides. The process implies breaking and reforming the ester bonds C–O–C that connect the fatty acid chains to the glycerol hubs of the fat molecules. The reactions involve catalysts, either inorganic chemicals or enzymes.

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.
SN2 Palmitate is a structured triglyceride where palmitic acid is bonded to the middle position (sn-2) of the glycerol backbone. Structured triglycerides are achieved through an enzymatic process using vegetable oils. Current usage of structured triglycerides is mainly for infant formula providing a human milk fat substitute.

Lipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.














