
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to relieve pain, fever, and inflammation. This includes painful menstrual periods, migraines, and rheumatoid arthritis. It may also be used to close a patent ductus arteriosus in a premature baby. It can be used by mouth or intravenously. It typically begins working within an hour.
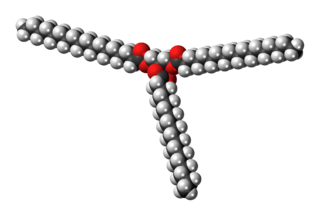
Stearin, or tristearin, or glyceryl tristearate is an odourless, white powder. It is a triglyceride derived from three units of stearic acid. Most triglycerides are derived from at least two and more commonly three different fatty acids. Like other triglycerides, stearin can crystallise in three polymorphs. For stearin, these melt at 54 (α-form), 65, and 72.5 °C (β-form).

Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation.

Propionic acid is a naturally occurring carboxylic acid with chemical formula CH
3CH
2CO
2H. It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion CH
3CH
2CO−
2 as well as the salts and esters of propionic acid are known as propionates or propanoates.

Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin.

Lead(II) sulfate (PbSO4) is a white solid, which appears white in microcrystalline form. It is also known as fast white, milk white, sulfuric acid lead salt or anglesite.

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D is 16:0. It is a major component of the oil from the fruit of oil palms, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats. Palmitates are the salts and esters of palmitic acid. The palmitate anion is the observed form of palmitic acid at physiologic pH (7.4).

Sodium acetate, CH3COONa, also abbreviated NaOAc, is the sodium salt of acetic acid. This colorless deliquescent salt has a wide range of uses.
Lauric acid, systematically dodecanoic acid, is a saturated fatty acid with a 12-carbon atom chain, thus having many properties of medium-chain fatty acids. It is a bright white, powdery solid with a faint odor of bay oil or soap. The salts and esters of lauric acid are known as laurates.
Myristic acid is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutmeg, from which it was first isolated in 1841 by Lyon Playfair.
This page provides supplementary chemical data on carbon dioxide.
Capric acid, also known as decanoic acid or decylic acid, is a saturated fatty acid, medium-chain fatty acid (MCFA), and carboxylic acid. Its formula is CH3(CH2)8COOH. Salts and esters of decanoic acid are called caprates or decanoates. The term capric acid is derived from the Latin "caper / capra" (goat) because the sweaty, unpleasant smell of the compound is reminiscent of goats.

Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid.

Trimyristin is a saturated fat and the triglyceride of myristic acid with the chemical formula C45H86O6. Trimyristin is a white to yellowish-gray solid that is insoluble in water, but soluble in ethanol, acetone, benzene, chloroform, dichloromethane, ether, and TBME.
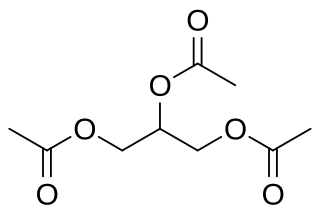
Triacetin, is the organic compound with the formula C3H5(OCOCH3)3. It is classified as a triglyceride, i.e., the triester of glycerol. It is a colorless, viscous, and odorless liquid with a high boiling point and a low melting point. It has a mild, sweet taste in concentrations lower than 500 ppm, but may appear bitter at higher concentrations. It is one of the glycerine acetate compounds.

n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent

Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.

Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers.
Tridecylic acid, or tridecanoic acid, is the organic compound with the formula CH3(CH2)11CO2H. It is a 13-carbon saturated fatty acid. It is a white solid.

















