
Forkhead box protein P2 (FOXP2) is a protein that, in humans, is encoded by the FOXP2 gene. FOXP2 is a member of the forkhead box family of transcription factors, proteins that regulate gene expression by binding to DNA. It is expressed in the brain, heart, lungs and digestive system.

The heritability of autism is the proportion of differences in expression of autism that can be explained by genetic variation; if the heritability of a condition is high, then the condition is considered to be primarily genetic. Autism has a strong genetic basis. Although the genetics of autism are complex, autism spectrum disorder (ASD) is explained more by multigene effects than by rare mutations with large effects.
22q13 deletion syndrome, also known as Phelan–McDermid syndrome (PMS), is a genetic disorder caused by deletions or rearrangements on the q terminal end of chromosome 22. Any abnormal genetic variation in the q13 region that presents with significant manifestations (phenotype) typical of a terminal deletion may be diagnosed as 22q13 deletion syndrome. There is disagreement among researchers as to the exact definition of 22q13 deletion syndrome. The Developmental Synaptopathies Consortium defines PMS as being caused by SHANK3 mutations, a definition that appears to exclude terminal deletions. The requirement to include SHANK3 in the definition is supported by many but not by those who first described 22q13 deletion syndrome.
Psychiatric genetics is a subfield of behavioral neurogenetics and behavioral genetics which studies the role of genetics in the development of mental disorders. The basic principle behind psychiatric genetics is that genetic polymorphisms are part of the causation of psychiatric disorders.

Transcription factor 7-like 2 , also known as TCF7L2 or TCF4, is a protein acting as a transcription factor that, in humans, is encoded by the TCF7L2 gene. The TCF7L2 gene is located on chromosome 10q25.2–q25.3, contains 19 exons. As a member of the TCF family, TCF7L2 can form a bipartite transcription factor and influence several biological pathways, including the Wnt signalling pathway.

Disrupted in schizophrenia 1 is a protein that in humans is encoded by the DISC1 gene. In coordination with a wide array of interacting partners, DISC1 has been shown to participate in the regulation of cell proliferation, differentiation, migration, neuronal axon and dendrite outgrowth, mitochondrial transport, fission and/or fusion, and cell-to-cell adhesion. Several studies have shown that unregulated expression or altered protein structure of DISC1 may predispose individuals to the development of schizophrenia, clinical depression, bipolar disorder, and other psychiatric conditions. The cellular functions that are disrupted by permutations in DISC1, which lead to the development of these disorders, have yet to be clearly defined and are the subject of current ongoing research. Although, recent genetic studies of large schizophrenia cohorts have failed to implicate DISC1 as a risk gene at the gene level, the DISC1 interactome gene set was associated with schizophrenia, showing evidence from genome-wide association studies of the role of DISC1 and interacting partners in schizophrenia susceptibility.

The Abelson helper integration site 1 (AHI1) is a protein coding gene that is known for the critical role it plays in brain development. Proper cerebellar and cortical development in the human brain depends heavily on AHI1. The AHI1 gene is prominently expressed in the embryonic hindbrain and forebrain. AHI1 specifically encodes the Jouberin protein and mutations in the expression of the gene is known to cause specific forms of Joubert syndrome. Joubert syndrome is autosomal recessive and is characterized by the brain malformations and mental retardation that AHI1 mutations have the potential to induce. AHI1 has also been associated with schizophrenia and autism due to the role it plays in brain development. An AHI1 heterozygous knockout mouse model was studied by Bernard Lerer and his group at Hadassah Medical Center in Jerusalem to elucidate the correlation between alterations in AHI1 expression and the pathogenesis of neuropsychiatric disorders. The core temperatures and corticosterone secretions of the heterozygous knockout mice after exposure to environmental and visceral stress exhibited extreme repression of autonomic nervous system and hypothalamic-pituitary-adrenal responses. The knockout mice demonstrated an increased resilience to different types of stress and these results lead to a correlation between emotional regulation and neuropsychiatric disorders.

Chromodomain-helicase-DNA-binding protein 8 is an enzyme that in humans is encoded by the CHD8 gene.

Neuregulin 3, also known as NRG3, is a neural-enriched member of the neuregulin protein family which in humans is encoded by the NRG3 gene. The NRGs are a group of signaling proteins part of the superfamily of epidermal growth factor, EGF like polypeptide growth factor. These groups of proteins possess an 'EGF-like domain' that consists of six cysteine residues and three disulfide bridges predicted by the consensus sequence of the cysteine residues.
In genetics, rs7341475 is a single nucleotide polymorphism (SNP) in the RELN gene that codes the reelin protein. The gene RELN is mapped to human chromosome 7 (7q22.1). The SNP rs7341475 is located in the fourth intron of RELN. The gene RELN has many more SNPs among its 65 exons and 64 introns, — even in intron 4 there are tens of SNPs.
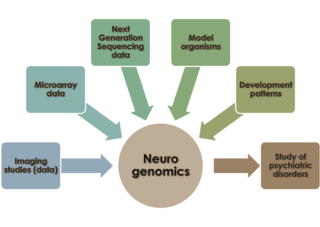
Neurogenomics is the study of how the genome of an organism influences the development and function of its nervous system. This field intends to unite functional genomics and neurobiology in order to understand the nervous system as a whole from a genomic perspective.
The Center for Applied Genomics is a research center at the Children's Hospital of Philadelphia that focuses on genomics research and the utilization of basic research findings in the development of new medical treatments.
Autism spectrum disorder (ASD) refers to a variety of conditions typically identified by challenges with social skills, communication, speech, and repetitive sensory-motor behaviors. The 11th International Classification of Diseases (ICD-11), released in January 2021, characterizes ASD by the associated deficits in the ability to initiate and sustain two-way social communication and restricted or repetitive behavior unusual for the individual's age or situation. Although linked with early childhood, the symptoms can appear later as well. Symptoms can be detected before the age of two and experienced practitioners can give a reliable diagnosis by that age. However, official diagnosis may not occur until much older, even well into adulthood. There is a large degree of variation in how much support a person with ASD needs in day-to-day life. This can be classified by a further diagnosis of ASD level 1, level 2, or level 3. Of these, ASD level 3 describes people requiring very substantial support and who experience more severe symptoms. ASD-related deficits in nonverbal and verbal social skills can result in impediments in personal, family, social, educational, and occupational situations. This disorder tends to have a strong correlation with genetics along with other factors. More research is identifying ways in which epigenetics is linked to autism. Epigenetics generally refers to the ways in which chromatin structure is altered to affect gene expression. Mechanisms such as cytosine regulation and post-translational modifications of histones. Of the 215 genes contributing, to some extent in ASD, 42 have been found to be involved in epigenetic modification of gene expression. Some examples of ASD signs are specific or repeated behaviors, enhanced sensitivity to materials, being upset by changes in routine, appearing to show reduced interest in others, avoiding eye contact and limitations in social situations, as well as verbal communication. When social interaction becomes more important, some whose condition might have been overlooked suffer social and other exclusion and are more likely to have coexisting mental and physical conditions. Long-term problems include difficulties in daily living such as managing schedules, hypersensitivities, initiating and sustaining relationships, and maintaining jobs.

Hermona Soreq is an Israeli professor of Molecular Neuroscience at The Hebrew University of Jerusalem. Best known for her work on the signaling of acetylcholine and its relevance in stress responses and neurodegenerative diseases such as Parkinson's and Alzheimer's.
Joseph D. Buxbaum is an American molecular and cellular neuroscientist, autism researcher, and the Director of the Seaver Autism Center at the Icahn School of Medicine at Mount Sinai. Buxbaum is also, along with Simon Baron-Cohen, the co-editor of the BioMed Central journal Molecular Autism, and is a member of the scientific advisory board of the Autism Science Foundation. Buxbaum is a Professor of Psychiatry, Neuroscience, and Genetics and Genomic Sciences. He is also the Vice Chair for Research and for Mentoring in the Department of Psychiatry at the Icahn School of Medicine at Mount Sinai.
Osnat Penn, born in 1981, is an Israeli computational biologist whose work focuses on molecular evolution, cell research, and immunoinformatics. She is the third Israeli scientist in three years to win the UNESCO-L’Oréal fellowship, which she received in 2013 for her work on the genetic origins of autism. Penn is currently a postdoctorate fellow at the University of Washington in Seattle, where she has been working since 2012.

Solute carrier family 39 member 12 is a protein that in humans is encoded by the SLC39A12 gene.

Ariel Darvasi was a Professor of Genetics and former Head of Life Sciences Studies and Vice Dean of the Faculty of Sciences at The Hebrew University of Jerusalem.

Snijders Blok–Campeau syndrome is a genetic disorder caused by mutations in the CHD3 gene. It is characterized by impaired intellectual development, macrocephaly, dysarthria and apraxia of speech, and certain distinctive facial features.

Beck–Fahrner syndrome, also known as BEFAHRS and TET3 deficiency, is a rare genetic disorder caused by mutations of the TET3 gene. It can occur de novo or can be inherited in an autosomal dominant manner. Mutations in the TET3 gene disrupts DNA demethylation during early embryogenesis and neural development. Most common clinical presentation includes global developmental delay, psychomotor retardation, neurodevelopmental disorders, hypotonia, epilepsy and dysmorphic features. It is diagnosed using molecular and genetic testing in setting of typical symptoms. Management is supportive and intended to improve quality of life.













