
A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. After invading a host cell's cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome, the reverse of the usual pattern, thus retro (backwards). The new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, transcribing and translating the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. Many retroviruses cause serious diseases in humans, other mammals, and birds.
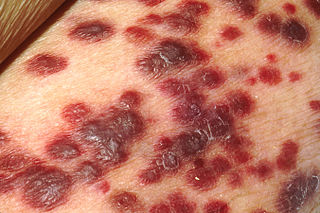
Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.
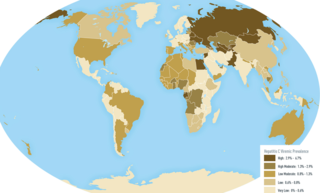
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.

Gammaretrovirus is a genus in the Retroviridae family. Example species are the murine leukemia virus and the feline leukemia virus. They cause various sarcomas, leukemias and immune deficiencies in mammals, reptiles and birds.
Rous sarcoma virus (RSV) is a retrovirus and is the first oncovirus to have been described. It causes sarcoma in chickens.

Viral transformation is the change in growth, phenotype, or indefinite reproduction of cells caused by the introduction of inheritable material. Through this process, a virus causes harmful transformations of an in vivo cell or cell culture. The term can also be understood as DNA transfection using a viral vector.

Seliciclib is an experimental drug candidate in the family of pharmacological cyclin-dependent kinase (CDK) inhibitors that preferentially inhibit multiple enzyme targets including CDK2, CDK7 and CDK9, which alter the growth phase or state within the cell cycle of treated cells. Seliciclib is being developed by Cyclacel.This is a phase II, dose ranging, multicenter, randomized, double-blind, placebo-controlled study.

A long terminal repeat (LTR) is a pair of identical sequences of DNA, several hundred base pairs long, which occur in eukaryotic genomes on either end of a series of genes or pseudogenes that form a retrotransposon or an endogenous retrovirus or a retroviral provirus. All retroviral genomes are flanked by LTRs, while there are some retrotransposons without LTRs. Typically, an element flanked by a pair of LTRs will encode a reverse transcriptase and an integrase, allowing the element to be copied and inserted at a different location of the genome. Copies of such an LTR-flanked element can often be found hundreds or thousands of times in a genome. LTR retrotransposons comprise about 8% of the human genome.
The gag-onc fusion protein is a general term for a fusion protein formed from a group-specific antigen ('gag') gene and that of an oncogene ('onc'), a gene that plays a role in the development of a cancer. The name is also written as Gag-v-Onc, with "v" indicating that the Onc sequence resides in a viral genome. Onc is a generic placeholder for a given specific oncogene, such as C-jun..
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.

Cyclin-dependent kinase 9 or CDK9 is a cyclin-dependent kinase associated with P-TEFb.

Cyclin-T1 is a protein that in humans is encoded by the CCNT1 gene.
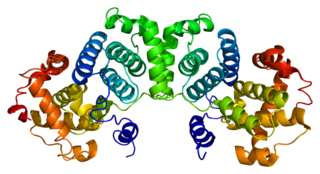
G2/mitotic-specific cyclin-B1 is a protein that in humans is encoded by the CCNB1 gene.

CDK-activating kinase assembly factor MAT1 is an enzyme that in humans is encoded by the MNAT1 gene.
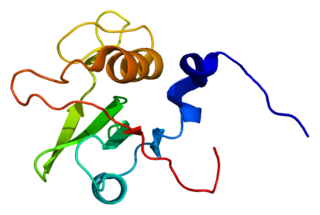
Tyrosine-protein kinase Fes/Fps also known as proto-oncogene c-Fes/Fps is an enzyme that in humans is encoded by the FES gene. FES was originally cloned as a retroviral oncogene from feline (v-FES) and avian (v-FPS) sarcomas. This triggered the subsequent identification and cloning of the cellular FES (c-FES) genes in birds and mammals.
The walleye epidermal hyperplasia viruses are two species of retroviruses classified under Epsilonretrovirus, a genus in the family of Retroviridae. There are three genome sequenced and identified exogenous retroviruses of this genus which include two known types associated with walleye epidermal hyperplasia disease. Both viral types are confirmed to be the causative agents of the neoplastic condition in the freshwater fish species, the North American walleye (Sander vitreus). The specific association of retroviral infection with proliferative lesions in fish is based on the presence of retrovirus-like particles and reverse transcriptase activity from neoplastic tissue. Although both virus types have been observed in lesions of diseased fish, each cell of the infected tissue is host to a specific virus. Transmission studies have also shown that WEHV-2 has been the more proliferative agent of the condition as compared to WEHV-1.
Retroviral matrix proteins are components of envelope-associated capsids of retroviruses. These proteins line the inner surface of viral envelopes and are associated with viral membranes.
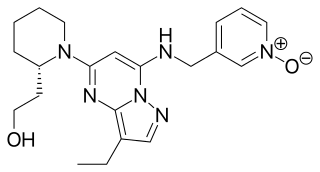
Dinaciclib (SCH-727965) is an experimental drug that inhibits cyclin-dependent kinases (CDKs). It is being evaluated in clinical trials for various cancer indications.
Gibbon-ape leukemia virus (GaLV) is an oncogenic, type C retrovirus that has been isolated from primate neoplasms, including the white-handed gibbon and woolly monkey. The virus was identified as the etiological agent of hematopoietic neoplasms, leukemias, and immune deficiencies within gibbons in 1971, during the epidemic of the late 1960s and early 1970s. Epidemiological research into the origins of GaLV has developed two hypotheses for the virus' emergence. These include cross-species transmission of the retrovirus present within a species of East Asian rodent or bat, and the inoculation or blood transfusion of a MbRV-related virus into captured gibbons populations housed at medical research institutions. The virus was subsequently identified in captive gibbon populations in Thailand, the US and Bermuda.
The Walleye dermal sarcoma virus (WDSV) is a retrovirus that infects walleye often causing oncogenesis. WDSV is an exogenous retrovirus belonging to the subfamily Orthoretrovirinae. This virus is related to the walleye epidermal hyperplasia viruses type 1 and type 2, all belonging to the epsilonretrovirus genus based on similarities of the gene coding for the reverse transcriptase conserved in retroviruses.













