
p53, also known as Tumor protein P53, cellular tumor antigen p53, or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins are crucial in vertebrates, where they prevent cancer formation. As such, p53 has been described as "the guardian of the genome" because of its role in conserving stability by preventing genome mutation. Hence TP53 is classified as a tumor suppressor gene.

A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or reduction in its function. In combination with other genetic mutations, this could allow the cell to grow abnormally. The loss of function for these genes may be even more significant in the development of human cancers, compared to the activation of oncogenes.

DnaJ homolog subfamily A member 3, mitochondrial, also known as Tumorous imaginal disc 1 (TID1), is a protein that in humans is encoded by the DNAJA3 gene on chromosome 16. This protein belongs to the DNAJ/Hsp40 protein family, which is known for binding and activating Hsp70 chaperone proteins to perform protein folding, degradation, and complex assembly. As a mitochondrial protein, it is involved in maintaining membrane potential and mitochondrial DNA (mtDNA) integrity, as well as cellular processes such as cell movement, growth, and death. Furthermore, it is associated with a broad range of diseases, including neurodegenerative diseases, inflammatory diseases, and cancers.

Inhibitor of growth protein 1 is a protein that in humans is encoded by the ING1 gene.

WW domain-containing oxidoreductase is an enzyme that in humans is encoded by the WWOX gene.

Retinoic acid receptor responder protein 3 is a protein that in humans is encoded by the RARRES3 gene.

Inhibitor of growth protein 2 is a protein that in humans is encoded by the ING2 gene.

Semaphorin-3B is a protein that in humans is encoded by the SEMA3B gene.

ETS homologous factor is a protein that in humans is encoded by the EHF gene. This gene encodes a protein that belongs to an ETS transcription factor subfamily characterized by epithelial-specific expression (ESEs). The encoded protein acts as a transcriptional repressor and may be associated with asthma susceptibility. This protein may be involved in epithelial differentiation and carcinogenesis.
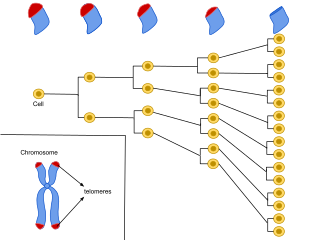
Cellular senescence is a phenomenon characterized by the cessation of cell division. In their experiments during the early 1960s, Leonard Hayflick and Paul Moorhead found that normal human fetal fibroblasts in culture reach a maximum of approximately 50 cell population doublings before becoming senescent. This process is known as "replicative senescence", or the Hayflick limit. Hayflick's discovery of mortal cells paved the path for the discovery and understanding of cellular aging molecular pathways. Cellular senescence can be initiated by a wide variety of stress inducing factors. These stress factors include both environmental and internal damaging events, abnormal cellular growth, oxidative stress, autophagy factors, among many other things.

Deleted in esophageal cancer 1 is a protein that in humans is encoded by the DEC1 gene.

Yippee-like 3 (Drosophila) is a protein that in humans is encoded by the YPEL3 gene. YPEL3 has growth inhibitory effects in normal and tumor cell lines. One of five family members (YPEL1-5), YPEL3 was named in reference to its Drosophila melanogaster orthologue. Initially discovered in a gene expression profiling assay of p53 activated MCF7 cells, induction of YPEL3 has been shown to trigger permanent growth arrest or cellular senescence in certain human normal and tumor cell types. DNA methylation of a CpG island near the YPEL3 promoter as well as histone acetylation may represent possible epigenetic mechanisms leading to decreased gene expression in human tumors.

The retinoblastoma protein is a tumor suppressor protein that is dysfunctional in several major cancers. One function of pRb is to prevent excessive cell growth by inhibiting cell cycle progression until a cell is ready to divide. When the cell is ready to divide, pRb is phosphorylated, inactivating it, and the cell cycle is allowed to progress. It is also a recruiter of several chromatin remodeling enzymes such as methylases and acetylases.

Joan Massagué, is a Spanish biologist and the current director of the Sloan Kettering Institute at Memorial Sloan Kettering Cancer Center. He is also an internationally recognized leader in the study of both cancer metastasis and growth factors that regulate cell behavior, as well as a professor at the Weill Cornell Graduate School of Medical Sciences.
Thomas J. Kelly is an American cancer researcher whose work focuses on the molecular mechanisms of DNA replication. Kelly is director of the Sloan-Kettering Institute, the basic research arm of the Memorial Sloan-Kettering Cancer Center. He holds the Center's Benno C. Schmidt Chair of Cancer Research.

Craig B. Thompson is an American cell biologist and a former president of the Memorial Sloan Kettering Cancer Center.
Professor Carol L. Prives FRS is the Da Costa Professor of Biological Sciences at Columbia University. She is known for her work in the characterisation of p53, an important tumor suppressor protein frequently mutated in cancer.
Robert Maki is an American medical oncologist, Professor of Medicine at the Hospital of the University of Pennsylvania, He is a specialist in the management of and translational research regarding sarcoma, the group of connective tissue malignancies that include leiomyosarcoma, gastrointestinal stromal tumor (GIST), liposarcoma, angiosarcoma, Ewing sarcoma, desmoid tumor and many others.
Viviane Tabar is an American neurosurgeon, the Chair of the Department of Neurosurgery at Memorial Sloan Kettering Cancer Center in New York since 2017.
Scott Neal Keeney is an American molecular biologist.













