
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. It converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using finely divided iron metal as a catalyst:

Methanol is an organic chemical compound and the simplest aliphatic alcohol, with the chemical formula CH3OH. It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol, but is more acutely toxic than the latter. Methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.
Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

Scripps Research is a nonprofit American medical research facility that focuses on research and education in the biomedical sciences. Headquartered in San Diego, California, the institute has over 170 laboratories employing 2,100 scientists, technicians, graduate students, and administrative and other staff.
The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.
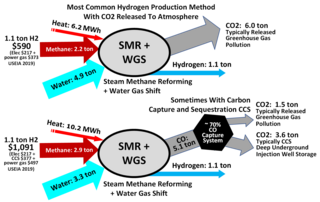
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of ammonia or methanol. The reaction is represented by this equilibrium:

Stranded gas is a natural gas field that has been discovered, but remains unusable for either physical or economic reasons. Gas found in an oil well is generally called associated gas rather than stranded gas but some flared gases from oil wells are stranded gases that are unusable due to economic reasons.
The water–gas shift reaction (WGSR) describes the reaction of carbon monoxide and water vapor to form carbon dioxide and hydrogen:

Gas to liquids (GTL) is a refinery process to convert natural gas or other gaseous hydrocarbons into longer-chain hydrocarbons, such as gasoline or diesel fuel. Methane-rich gases are converted into liquid synthetic fuels. Two general strategies exist: (i) direct partial combustion of methane to methanol and (ii) Fischer–Tropsch-like processes that convert carbon monoxide and hydrogen into hydrocarbons. Strategy ii is followed by diverse methods to convert the hydrogen-carbon monoxide mixtures to liquids. Direct partial combustion has been demonstrated in nature but not replicated commercially. Technologies reliant on partial combustion have been commercialized mainly in regions where natural gas is inexpensive.

Methyl bisulfate is a chemical compound with the molecular formula (CH3)HSO4. This compound is the mono-methyl ester of sulfuric acid. Its structure is CH3−O−S(=O)2−OH. The significance of methyl bisulfate is that it is an intermediate in the hydrolysis of the important reagent dimethyl sulfate, (CH3)2SO4:
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Most hydrogen is gray hydrogen made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as blue hydrogen.

In organic chemistry and organometallic chemistry, carbon–hydrogen bond activation is a type of organic reaction in which a carbon–hydrogen bond is cleaved and replaced with a C−X bond. Some authors further restrict the term C–H activation to reactions in which a C–H bond, one that is typically considered to be "unreactive", interacts with a transition metal center M, resulting in its cleavage and the generation of an organometallic species with an M–C bond. The intermediate of this step could then undergo subsequent reactions with other reagents, either in situ or in a separate step, to produce the functionalized product.

Methane is a chemical compound with the chemical formula CH4. It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an organic compound, and among the simplest of organic compounds. Methane is also a hydrocarbon.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.

Lanny D. Schmidt was an American chemist, inventor, author, and Regents Professor of Chemical Engineering and Materials Science at the University of Minnesota. He is well known for his extensive work in surface science, detailed chemistry (microkinetics), chemical reaction engineering, catalysis, and renewable energy. He is also well known for mentoring over a hundred graduate students and his work on millisecond reactors and reactive flash volatilization.
The oxidative coupling of methane (OCM) is a potential chemical reaction studied in the 1980s for the direct conversion of natural gas, primarily consisting of methane, into value-added chemicals. Although the reaction would have strong economics if practicable, no effective catalysts are known, and thermodynamic arguments suggest none can exist.
The first time a catalyst was used in the industry was in 1746 by J. Roebuck in the manufacture of lead chamber sulfuric acid. Since then catalysts have been in use in a large portion of the chemical industry. In the start only pure components were used as catalysts, but after the year 1900 multicomponent catalysts were studied and are now commonly used in the industry.

Roy A. Periana is a Guyanese-American organometallic chemist.
Methane functionalization is the process of converting methane in its gaseous state to another molecule with a functional group, typically methanol or acetic acid, through the use of transition metal catalysts.

2,2′-Bipyrimidine is an organic compound with the formula (C4H3N2)2. It is a derivative of the heterocycle pyrimidine. It is a white solid. The compound is used as a bridging ligand in coordination chemistry.














