This article needs additional citations for verification .(October 2022) |
Secondary is a term used in organic chemistry to classify various types of compounds (e. g. alcohols, https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Alcohols/Nomenclature_of_Alcohols alkyl halides, amines) or reactive intermediates (e. g. alkyl radicals, carbocations). An atom is considered secondary if it has two 'R' Groups attached to it. [1] An 'R' group is a carbon containing group such as a methyl (CH3). A secondary compound is most often classified on an alpha carbon (middle carbon) or a nitrogen. The word secondary comes from the root word 'second' which means two.
Contents
- Secondary alcohols
- Secondary amines
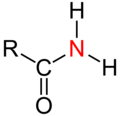
- Secondary amides
- Secondary phosphines
- Further uses
- See also
- References
| Red highlighted central atoms in various groups of chemical compounds. Secondary central atoms compared with primary, tertiary and quaternary central atoms. | ||||
| primary | secondary | tertiary | quaternary | |
| Carbon atom in an alkane |  |  |  |  |
This nomenclature can be used in many cases and further used to explain relative reactivity. The reactivity of molecules varies with respect to the attached atoms. Thus, a primary, secondary, tertiary and quaternary molecule of the same function group will have different reactivities.