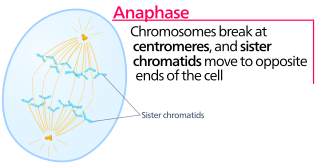
Anaphase, is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes are moved to opposite poles of the cell. Chromosomes also reach their overall maximum condensation in late anaphase, to help chromosome segregation and the re-formation of the nucleus.

Telophase is the final stage in both meiosis and mitosis in a eukaryotic cell. During telophase, the effects of prophase and prometaphase are reversed. As chromosomes reach the cell poles, a nuclear envelope is re-assembled around each set of chromatids, the nucleoli reappear, and chromosomes begin to decondense back into the expanded chromatin that is present during interphase. The mitotic spindle is disassembled and remaining spindle microtubules are depolymerized. Telophase accounts for approximately 2% of the cell cycle's duration.
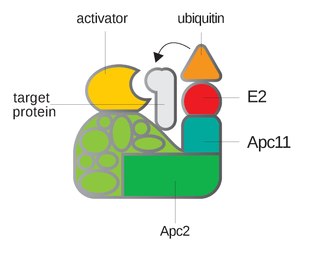
Anaphase-promoting complex is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin (Apc2) and RING (Apc11) subunit much like SCF. Other parts of the APC/C have unknown functions but are highly conserved.
Maturation-promoting factor (abbreviated MPF, also called mitosis-promoting factor or M-Phase-promoting factor) is the cyclin-Cdk complex that was discovered first in frog eggs. It stimulates the mitotic and meiotic phases of the cell cycle. MPF promotes the entrance into mitosis (the M phase) from the G2 phase by phosphorylating multiple proteins needed during mitosis. MPF is activated at the end of G2 by a phosphatase, which removes an inhibitory phosphate group added earlier.

The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), or the mitotic checkpoint, is a cell cycle checkpoint during mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles. Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together.
G2 phase, Gap 2 phase, or Growth 2 phase, is the third subphase of interphase in the cell cycle directly preceding mitosis. It follows the successful completion of S phase, during which the cell’s DNA is replicated. G2 phase ends with the onset of prophase, the first phase of mitosis in which the cell’s chromatin condenses into chromosomes.

Separase, also known as separin, is a cysteine protease responsible for triggering anaphase by hydrolysing cohesin, which is the protein responsible for binding sister chromatids during the early stage of anaphase. In humans, separin is encoded by the ESPL1 gene.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.
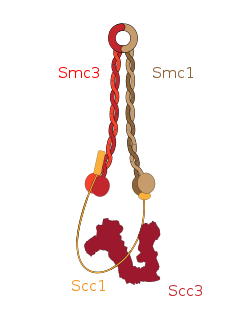
Cohesin is a protein complex that mediates sister chromatid cohesion, homologous recombination and DNA looping. Cohesin is formed of SMC3, SMC1, SCC1 and SCC3. Cohesin holds sister chromatids together after DNA replication until anaphase when removal of cohesin leads to separation of sister chromatids. The complex forms a ring-like structure and it is believed that sister chromatids are held together by entrapment inside the cohesin ring. Cohesin is a member of the SMC family of protein complexes which includes Condensin, MukBEF and SMC-ScpAB.
Mad2 is an essential spindle checkpoint protein. The spindle checkpoint system is a regulatory system that restrains progression through the metaphase-to-anaphase transition. The Mad2 gene was first identified in the yeast S. cerevisiae in a screen for genes which when mutated would confer sensitivity to microtubule poisons. The human orthologues of Mad2 were first cloned in a search for human cDNAs that would rescue the microtubule poison-sensitivity of a yeast strain in which a kinetochore binding protein was missing. The protein was shown to be present at unattached kinetochores and antibody inhibition studies demonstrated it was essential to execute a block in the metaphase-to-anaphase transition in response to the microtubule poison nocodazole. Subsequent cloning of the Xenopus laevis orthologue, facilitated by the sharing of the human sequence, allowed for the characterization of the mitotic checkpoint in egg extracts.
Polo-like kinases (Plks) are regulatory serine/threonin kinases of the cell cycle involved in mitotic entry, mitotic exit, spindle formation, cytokinesis, and meiosis. Only one Plk is found in the genomes of fruit flies (Polo), budding yeast (Cdc5) and fission yeast (Plo1). Vertebrates, however, have many Plk family members including Plk1, Plk2/Snk, Plk3/Prk/FnK, Plk4/Sak and Plk5. Of the vertebrate Plk family members, the mammalian Plk1 has been most extensively studied. During mitosis and cytokinesis, Plks associate with several structures including the centrosome, kinetochores, and the central spindle.

The cell division cycle protein 20 homolog is an essential regulator of cell division that is encoded by the CDC20 gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex (APC/C), a large 11-13 subunit complex that initiates chromatid separation and entrance into anaphase. The APC/CCdc20 protein complex has two main downstream targets. Firstly, it targets securin for destruction, enabling the eventual destruction of cohesin and thus sister chromatid separation. It also targets S and M-phase (S/M) cyclins for destruction, which inactivates S/M cyclin-dependent kinases (Cdks) and allows the cell to exit from mitosis. A closely related protein, Cdc20homologue-1 (Cdh1) plays a complementary role in the cell cycle.

Mitotic checkpoint protein BUB3 is a protein that in humans is encoded by the BUB3 gene.
A series of biochemical switches control transitions between and within the various phases of the cell cycle. The cell cycle is a series of complex, ordered, sequential events that control how a single cell divides into two cells, and involves several different phases. The phases include the G1 and G2 phases, DNA replication or S phase, and the actual process of cell division, mitosis or M phase. During the M phase, the chromosomes separate and cytokinesis occurs.
Cdc14 and Cdc14 are a gene and its protein product respectively. Cdc14 is found in most of the eukaryotes. Cdc14 was defined by Hartwell in his famous screen for loci that control the cell cycle of Saccharomyces cerevisiae. Cdc14 was later shown to encode a protein phosphatase. Cdc14 is dual-specificity, which means it has serine/threonine and tyrosine-directed activity. A preference for serines next to proline is reported. Many early studies, especially in the budding yeast Saccharomyces cerevisiae, demonstrated that the protein plays a key role in regulating late mitotic processes. However, more recent work in a range of systems suggests that its cellular function is more complex.

Cdh1 is one of the substrate adaptor protein of the anaphase-promoting complex (APC) in the budding yeast Saccharomyces cerevisiae. Functioning as an activator of the APC/C, Cdh1 regulates the activity and substrate specificity of this ubiquitin E3-ligase. The human homolog is encoded by the FZR1 gene, which is not to be confused with the CDH1 gene.
Clb5 and Clb6 are B-type, S-phase cyclins in yeast that assist in cell cycle regulation. Clb5 and Clb6 bind and activate Cdk1, and high levels of these cyclins are required for entering S-phase. S-phase cyclin binding to Cdk1 directly stimulates DNA replication as well as progression to the next phase of the cell cycle.
Mitotic Exit is an important transition point that signifies the end of mitosis and the onset of new G1 phase for a cell, and the cell needs to rely on specific control mechanisms to ensure that once it exits mitosis, it never returns to mitosis until it has gone through G1, S, and G2 phases and passed all the necessary checkpoints. Many factors including cyclins, cyclin-dependent kinases (CDKs), ubiquitin ligases, inhibitors of cyclin-dependent kinases, and reversible phosphorylations regulate mitotic exit to ensure that cell cycle events occur in correct order with fewest errors. The end of mitosis is characterized by spindle breakdown, shortened kinetochore microtubules, and pronounced outgrowth of astral (non-kinetochore) microtubules. For a normal eukaryotic cell, mitotic exit is irreversible.

Frank Uhlmann FRS is a group leader at the Francis Crick Institute in London.

The anaphase- promoting complex or cyclosome (APC/C) is a highly specific ubiquitin protein ligase responsible for triggering events of late mitosis. In early mitosis, Cdc20 levels rise and APC/C binds to form active APC/CCdc20. This then leads to the destruction of mitotic cyclins, securin, and other proteins to trigger chromosome separation in anaphase. In early anaphase, Cdk1 is inactivated, leading to the activation of Cdh1, the other activator subunit of APC/C. This then triggers the degradation of Cdc20 and leads to the activation of APC/CCdh1 through G1 to suppress S- phase cyclin-Cdk activity. At the end of G1, APC/CCdh1 is inactivated and S- phase and mitotic cyclins gets reaccumulate as the cell progresses to S phase.















