Related Research Articles

In medicine, dialysis is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform these functions naturally. This is referred to as renal replacement therapy. The first successful dialysis was performed in 1943.

Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis—or occasionally by more specialized processes of facilitated diffusion, passive transport or active transport. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials thicker than a membrane are also semipermeable. One example of this is the thin film on the inside of the egg. Note that a semipermeable membrane is not the same as a selectively permeable membrane. Semipermeable membrane describes a membrane that allows some particles to pass through, whereas the selectively permeable membrane "chooses" what passes through.

Water quality refers to the chemical, physical, and biological characteristics of water based on the standards of its usage. It is most frequently used by reference to a set of standards against which compliance, generally achieved through treatment of the water, can be assessed. The most common standards used to monitor and assess water quality convey the health of ecosystems, safety of human contact, and condition of drinking water. Water quality has a significant impact on water supply and oftentimes determines supply options.

Water pollution is the contamination of water bodies, usually as a result of human activities. Water bodies include for example lakes, rivers, oceans, aquifers and groundwater. Water pollution results when contaminants are introduced into the natural environment. For example, releasing inadequately treated wastewater into natural water bodies can lead to degradation of aquatic ecosystems. In turn, this can lead to public health problems for people living downstream. They may use the same polluted river water for drinking or bathing or irrigation. Water pollution is the leading worldwide cause of death and disease, e.g. due to water-borne diseases.

Environmental remediation deals with the removal of pollution or contaminants from environmental media such as soil, groundwater, sediment, or surface water. Remedial action is generally subject to an array of regulatory requirements, and may also be based on assessments of human health and ecological risks where no legislative standards exist, or where standards are advisory.
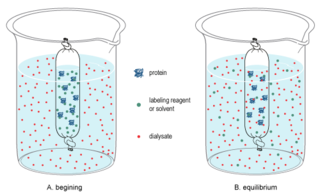
In biochemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.

Hemodialysis, also spelled haemodialysis, or simply dialysis, is a process of purifying the blood of a person whose kidneys are not working normally. This type of dialysis achieves the extracorporeal removal of waste products such as creatinine and urea and free water from the blood when the kidneys are in a state of kidney failure. Hemodialysis is one of three renal replacement therapies. An alternative method for extracorporeal separation of blood components such as plasma or cells is apheresis.

Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

Microdialysis is a minimally-invasive sampling technique that is used for continuous measurement of free, unbound analyte concentrations in the extracellular fluid of virtually any tissue. Analytes may include endogenous molecules to assess their biochemical functions in the body, or exogenous compounds to determine their distribution within the body. The microdialysis technique requires the insertion of a small microdialysis catheter into the tissue of interest. The microdialysis probe is designed to mimic a blood capillary and consists of a shaft with a semipermeable hollow fiber membrane at its tip, which is connected to inlet and outlet tubing. The probe is continuously perfused with an aqueous solution (perfusate) that closely resembles the (ionic) composition of the surrounding tissue fluid at a low flow rate of approximately 0.1-5μL/min. Once inserted into the tissue or (body)fluid of interest, small solutes can cross the semipermeable membrane by passive diffusion. The direction of the analyte flow is determined by the respective concentration gradient and allows the usage of microdialysis probes as sampling as well as delivery tools. The solution leaving the probe (dialysate) is collected at certain time intervals for analysis.
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to remove ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the pores (holes), but should allow smaller components of the solution (such as solvent molecules, i.e., water, H2O) to pass freely.
Environmental monitoring describes the processes and activities that need to take place to characterize and monitor the quality of the environment. Environmental monitoring is used in the preparation of environmental impact assessments, as well as in many circumstances in which human activities carry a risk of harmful effects on the natural environment. All monitoring strategies and programs have reasons and justifications which are often designed to establish the current status of an environment or to establish trends in environmental parameters. In all cases, the results of monitoring will be reviewed, analyzed statistically, and published. The design of a monitoring program must therefore have regard to the final use of the data before monitoring starts.
T.E. Laboratories (TelLab), based in Tullow, Ireland, holds the global licence to manufacture and sell Chemcatcher®. Chemcatcher® is a passive sampling device for monitoring a variety of pollutants in water. Most monitoring programmes involve the periodic collection of low volume spot samples of water, which is challenging, particularly where levels fluctuate over time and when chemicals are only present at trace, yet toxicologically relevant concentrations. Chemcatcher® is used to measure time-weighted average (TWA) or equilibrium concentrations of a wide range of pollutants in water. This allows the end user to obtain a more representative picture of the chemicals that may be present in the aquatic environment. The Chemcatcher® concept was developed by Professors Richard Greenwood and Graham Mills at the University of Portsmouth, together with colleagues from Chalmers University of Technology, Sweden. The device is patented in a number of countries and the name is a registered trademark in Ireland and the United Kingdom
Bioconcentration is the accumulation of a chemical in or on an organism when the source of chemical is solely water. Bioconcentration is a term that was created for use in the field of aquatic toxicology. Bioconcentration can also be defined as the process by which a chemical concentration in an aquatic organism exceeds that in water as a result of exposure to a waterborne chemical.
Analytical thermal desorption, known within the analytical chemistry community simply as "thermal desorption" (TD), is a technique that concentrates volatile organic compounds (VOCs) in gas streams prior to injection into a gas chromatograph (GC). It can be used to lower the detection limits of GC methods, and can improve chromatographic performance by reducing peak widths.
A polar organic chemical integrative sampler (POCIS) is a passive sampling device which allows for the in situ collection of a time-integrated average of hydrophilic organic contaminants developed by researchers with the United States Geological Survey in Columbia, Missouri. POCIS provides a means for estimating the toxicological significance of waterborne contaminants. The POCIS sampler mimics the respiratory exposure of organisms living in the aquatic environment and can provide an understanding of bioavailable contaminants present in the system. POCIS can be deployed in a wide range of aquatic environments and is commonly used to assist in environmental monitoring studies.
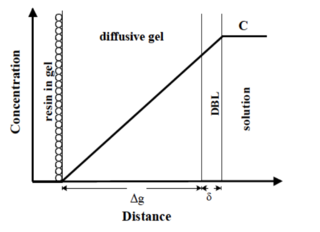
The diffusive gradients in thin films (DGT) technique is an environmental chemistry technique for the detection of elements and compounds in aqueous environments, including natural waters, sediments and soils. It is well suited to in situ detection of bioavailable toxic trace metal contaminants. The technique involves using a specially-designed passive sampler that houses a binding gel, diffusive gel and membrane filter. The element or compound passes through the membrane filter and diffusive gel and is assimilated by the binding gel in a rate-controlled manner. Post-deployment analysis of the binding gel can be used to determine the time-weighted-average bulk solution concentration of the element or compound via a simple equation.
The stabilized liquid membrane device (SLMD) is a passive, integrative sampler that provides an alternative or complementary approach to the conventional water sampling of aqueous metals. The simple device is composed of nonporous low-density plastic lay-flat tubing, which is filled with a chemical mixture containing a chelating agent and a long chain organic acid. The water-insoluble chelating agent-organic acid mixture diffuses in a controlled manner to the exterior surface of the sampler membrane and binds to environmental metals. In practice, the SLMD provides for continuous sequestration of bioavailable forms of trace metals, such as, cadmium (Cd), cobalt (Co), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). The SLMD can also be utilized for in-laboratory preconcentration and speciation of bioavailable trace metals from grab water samples.
The Diamond Head Oil Refinery is a former oil reprocessing facility located in Kearny, New Jersey, United States, that was designated as a Superfund site by the Environmental Protection Agency (EPA). It opened up in 1946, but then stopped production in 1979 and has been inactive since then. The refinery was shut down in 1980 and the EPA designated it as a Superfund site in 1991 due to the discovery of toxic chemicals in the soil and the surface water. This created a dangerous work environment for the workers at the facility. The EPA proposed a clean up plan for the site, but it has yet to take effect. So far, the Diamond Head site is still in the process of being cleaned up. Although cleanup plans were discussed and finalized, the future of the Diamond Head Oil Refinery and its cleanup state is unknown.

A stabilized liquid membrane device or SLMD is a type of passive sampling device which allows for the in situ, integrative collection of waterborne, labile ionic metal contaminants. By capturing and sequestering metal ions onto its surface continuously over a period of days to weeks, an SLMD can provide an integrative measurement of bioavailable toxic metal ions present in the aqueous environment. As such, they have been used in conjunction with other passive samplers in ecological field studies.
Passive sampling is an environmental monitoring technique involving the use of a collecting medium, such as a man-made device or biological organism, to accumulate chemical pollutants in the environment over time. This is in contrast to grab sampling, which involves taking a sample directly from the media of interest at one point in time. In passive sampling, average chemical concentrations are calculated over a device's deployment time, which avoids the need to visit a sampling site multiple times to collect multiple representative samples. Currently, passive samplers have been developed and deployed to detect toxic metals, pesticides, pharmaceuticals, radionuclides, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and other organic compounds in water, while some passive samplers can detect hazardous substances in the air.
References
- 1 2 Huckins, James N.; Manuweera, Gamini K.; Petty, Jimmie D.; MacKay, Donald; Lebo, Jon A. (December 12, 1993). "Lipid-containing Semipermeable Membrane Devices for Monitoring Organic Contaminants in Water". Environmental Science & Technology. 27 (12): 2489–2496. Bibcode:1993EnST...27.2489H. doi:10.1021/es00048a028.
- 1 2 3 Alvarez, David (2010). Guidelines for the Use of the Semipermeable Membrane Device (SPMD) and the Polar Organic Chemical Integrative Sampler (POCIS) in Environmental Monitoring Studies. USGS.
- 1 2 3 4 5 6 7 8 "Semipermeable Membrane Device". NOAA. April 16, 2013.
- 1 2 Guidelines for Using Passive Samplers to Monitor Organic Contaminants. EPA. 2012.
- 1 2 Huff, Tom (2016). Semipermeable Membrane Device. USGS.
- ↑ Foote, Eric. "Using SPMDs to Assess Natural Recovery of PCB-contaminated Sediments in Lake Hartwell, SC: I. A Field Test of New In-Situ Deployment Methods". Soil and Sediment Contamination.
- ↑ Superfund Records Center. EPA. 2005.