Functional electrical stimulation (FES) is a technique that uses low-energy electrical pulses to artificially generate body movements in individuals who have been paralyzed due to injury to the central nervous system. More specifically, FES can be used to generate muscle contraction in otherwise paralyzed limbs to produce functions such as grasping, walking, bladder voiding and standing. This technology was originally used to develop neuroprostheses that were implemented to permanently substitute impaired functions in individuals with spinal cord injury (SCI), head injury, stroke and other neurological disorders. In other words, a person would use the device each time he or she wanted to generate a desired function. FES is sometimes also referred to as neuromuscular electrical stimulation (NMES).
Neuroprosthetics is a discipline related to neuroscience and biomedical engineering concerned with developing neural prostheses. They are sometimes contrasted with a brain–computer interface, which connects the brain to a computer rather than a device meant to replace missing biological functionality.

Electrosurgery is the application of a high-frequency alternating polarity, electrical current to biological tissue as a means to cut, coagulate, desiccate, or fulgurate tissue.. Its benefits include the ability to make precise cuts with limited blood loss. Electrosurgical devices are frequently used during surgical operations helping to prevent blood loss in hospital operating rooms or in outpatient procedures.
In neuroscience, single-unit recordings provide a method of measuring the electro-physiological responses of single neurons using a microelectrode system. When a neuron generates an action potential, the signal propagates down the neuron as a current which flows in and out of the cell through excitable membrane regions in the soma and axon. A microelectrode is inserted into the brain, where it can record the rate of change in voltage with respect to time. These microelectrodes must be fine-tipped, high-impedance conductors; they are primarily glass micro-pipettes or metal microelectrodes made of platinum or tungsten. Microelectrodes can be carefully placed close to the cell membrane, allowing the ability to record extracellularly.

Chronaxie is the minimum time required for an electric current to double the strength of the rheobase to stimulate a muscle or a neuron. Rheobase is the lowest intensity with indefinite pulse duration which just stimulated muscles or nerves. Chronaxie is dependent on the density of voltage-gated sodium channels in the cell, which affect that cell’s excitability. Chronaxie varies across different types of tissue: fast-twitch muscles have a lower chronaxie, slow-twitch muscles have a higher one. Chronaxie is the tissue-excitability parameter that permits choice of the optimum stimulus pulse duration for stimulation of any excitable tissue. Chronaxie (c) is the Lapicque descriptor of the stimulus pulse duration for a current of twice rheobasic (b) strength, which is the threshold current for an infinitely long-duration stimulus pulse. Lapicque showed that these two quantities (c,b) define the strength-duration curve for current: I = b(1+c/d), where d is the pulse duration. However, there are two other electrical parameters used to describe a stimulus: energy and charge. The minimum energy occurs with a pulse duration equal to chronaxie. Minimum charge (bc) occurs with an infinitely short-duration pulse. Choice of a pulse duration equal to 10c requires a current of only 10% above rheobase (b). Choice of a pulse duration of 0.1c requires a charge of 10% above the minimum charge (bc).
Local field potentials (LFP) are transient electrical signals generated in nervous and other tissues by the summed and synchronous electrical activity of the individual cells in that tissue. LFP are "extracellular" signals, meaning that they are generated by transient imbalances in ion concentrations in the spaces outside the cells, that result from cellular electrical activity. LFP are 'local' because they are recorded by an electrode placed nearby the generating cells. As a result of the Inverse-square law, such electrodes can only 'see' potentials in spatially limited radius. They are 'potentials' because they are generated by the voltage that results from charge separation in the extracellular space. They are 'field' because those extracellular charge separations essentially create a local electric field. LFP are typically recorded with a high-impedance microelectrode placed in the midst of the population of cells generating it. They can be recorded, for example, via a microelectrode placed in the brain of an anesthetized animal, or in an in vitro brain thin slice.
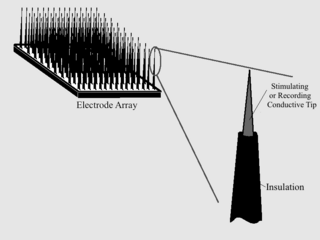
Microelectrode arrays (MEAs) are devices that contain multiple microelectrodes through which neural signals are obtained or delivered, essentially serving as neural interfaces that connect neurons to electronic circuitry. There are two general classes of MEAs: implantable MEAs, used in vivo, and non-implantable MEAs, used in vitro.
John Peter Wikswo, Jr. is a biological physicist at Vanderbilt University. He was born in Lynchburg, Virginia, United States.
A depolarizing prepulse (DPP) is an electrical stimulus that causes the potential difference measured across a neuronal membrane to become more positive or less negative, and precedes another electrical stimulus. DPPs may be of either the voltage or current stimulus variety and have been used to inhibit neural activity, selectively excite neurons, and increase the pain threshold associated with electrocutaneous stimulation.
Neurostimulation is the purposeful modulation of the nervous system's activity using invasive or non-invasive means. Neurostimulation usually refers to the electromagnetic approaches to neuromodulation.
A hippocampus prosthesis is a type of cognitive prosthesis. Prosthetic devices replace normal function of a damaged body part; this can be simply a structural replacement or a rudimentary, functional replacement. However, prosthetics involving the brain have some special categories and requirements. "Input" prosthetics, such as retinal or cochlear implant, supply signals to the brain that the patient eventually learns to interpret as sight or sound. "Output" prosthetics use brain signals to drive a bionic arm, hand or computer device, and require considerable training during which the patient learns to generate the desired action via their thoughts. Both of these types of prosthetics rely on the plasticity of the brain to adapt to the requirement of the prosthesis, thus allowing the user to "learn" the use of his new body part. A cognitive or "brain-to-brain" prosthesis involves neither learned input nor output signals, but the native signals used normally by the area of the brain to be replaced. Thus, such a device must be able to fully replace the function of a small section of the nervous system—using that section's normal mode of operation. In order to achieve this, developers require a deep understanding of the functioning of the nervous system. The scope of design must include a reliable mathematical model as well as the technology in order to properly manufacture and install a cognitive prosthesis. The primary goal of an artificial hippocampus is to provide a cure for Alzheimer's disease and other hippocampus—related problems. To do so, the prosthesis has to be able to receive information directly from the brain, analyze the information and give an appropriate output to the cerebral cortex; in other words, it must behave just like a natural hippocampus. At the same time, the artificial organ must be completely autonomous, since any exterior power source will greatly increase the risk of infection.

Platinum-iridium alloys are alloys of the platinum group precious metals platinum and iridium.
As with any material implanted in the body, it is important to minimize or eliminate foreign body response and maximize effectual integration. Neural implants have the potential to increase the quality of life for patients with such disabilities as Alzheimer's, Parkinson's, epilepsy, depression, and migraines. With the complexity of interfaces between a neural implant and brain tissue, adverse reactions such as fibrous tissue encapsulation that hinder the functionality, occur. Surface modifications to these implants can help improve the tissue-implant interface, increasing the lifetime and effectiveness of the implant.
A peripheral nerve interface is the bridge between the peripheral nervous system and a computer interface which serves as a bi‐directional information transducer recording and sending signals between the human body and a machine processor. Interfaces to the nervous system usually take the form of electrodes for stimulation and recording, though chemical stimulation and sensing are possible. Research in this area is focused on developing peripheral nerve interfaces for the restoration of function following disease or injury to minimize associated losses. Peripheral nerve interfaces also enable electrical stimulation and recording of the peripheral nervous system to study the form and function of the peripheral nervous system. Many researchers also focus in the area of neuroprosthesis, linking the human nervous system to bionics in order to mimic natural sensorimotor control and function. Successful implantation of peripheral nerve interfaces depend on a number of factors which include appropriate indication, perioperative testing, differentiated planning, and functional training. Typically microelectrode devices are implanted adjacent to, around or within the nerve trunk to establish contact with the peripheral nervous system. Different approaches may be used depending on the type of signal desired and attainable.
A chronic electrode implant is an electronic device implanted chronically into the brain or other electrically excitable tissue. It may record electrical impulses in the brain or may stimulate neurons with electrical impulses from an external source.
A cortical implant is a subset of neuroprosthetics that is in direct connection with the cerebral cortex of the brain. By directly interfacing with different regions of the cortex, the cortical implant can provide stimulation to an immediate area and provide different benefits, depending on its design and placement. A typical cortical implant is an implantable microelectrode array, which is a small device through which a neural signal can be received or transmitted.

Ear-EEG is a method for measuring dynamics of brain activity through the minute voltage changes observable on the skin, typically by placing electrodes on the scalp. In ear-EEG, the electrodes are exclusively placed in or around the outer ear, resulting in both a much greater invisibility and wearer mobility compared to full scalp electroencephalography (EEG), but also significantly reduced signal amplitude, as well as reduction in the number of brain regions in which activity can be measured. It may broadly be partitioned into two groups: those using electrode positions exclusively within the concha and ear canal, and those also placing electrodes close to the ear, usually hidden behind the ear lobe. Generally speaking, the first type will be the most invisible, but also offer the most challenging (noisy) signal. Ear-EEG is a good candidate for inclusion in a hearable device, however, due to the high complexity of ear-EEG sensors, this has not yet been done.
Neural dust is a term used to refer to millimeter-sized devices operated as wirelessly powered nerve sensors; it is a type of brain–computer interface. The sensors may be used to study, monitor, or control the nerves and muscles and to remotely monitor neural activity. In practice, a medical treatment could introduce thousands of neural dust devices into human brains. The term is derived from "smart dust", as the sensors used as neural dust may also be defined by this concept.