Related Research Articles

A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding.
Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons.
In chemistry, the mesomeric effect is a property of substituents or functional groups in a chemical compound. It is defined as the polarity produced in the molecule by the interaction of two pi bonds or between a pi bond and lone pair of electrons present on an adjacent atom. This change in electron arrangement results in the formation of resonance structures that hybridize into the molecule's true structure. The pi electrons then move away from or toward a particular substituent group. The mesomeric effect is stronger in compounds with a lower ionization potential. This is because the electron transfer states will have lower energies.
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into a resonance hybrid in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical contributing structures.
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed.
In chemistry, homolysis or homolytic fission is the dissociation of a molecular bond by a process where each of the fragments retains one of the originally bonded electrons. During homolytic fission of a neutral molecule with an even number of electrons, two radicals will be generated. That is, the two electrons involved in the original bond are distributed between the two fragment species. Bond cleavage is also possible by a process called heterolysis.

In organic chemistry, hyperconjugation refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed negative hyperconjugation. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in another atom directly attached to it. However, extended versions of hyperconjugation can be important as well. The Baker–Nathan effect, sometimes used synonymously for hyperconjugation, is a specific application of it to certain chemical reactions or types of structures.
In Organic chemistry, the inductive effect in a molecule is a local change in the electron density due to electron-withdrawing or electron-donating groups elsewhere in the molecule, resulting in a permanent dipole in a bond. It is present in a σ (sigma) bond, unlike the electromeric effect which is present in a π (pi) bond.
In chemistry, Bent's rule describes and explains the relationship between the orbital hybridization and the electronegativities of substituents. The rule was stated by Henry A. Bent as follows:
Atomic s character concentrates in orbitals directed toward electropositive substituents.

In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less-hindered equatorial orientation that would be expected from steric considerations. This effect was originally observed in pyranose rings by J. T. Edward in 1955 when studying carbohydrate chemistry.
An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on fundamental chemical processes such as acid-base reactions, redox potentials, and substitution reactions.
In organic chemistry, the Hammett equation describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a reaction constant. This equation was developed and published by Louis Plack Hammett in 1937 as a follow-up to qualitative observations in his 1935 publication.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
An electric effect influences the structure, reactivity, or properties of a molecule but is neither a traditional bond nor a steric effect. In organic chemistry, the term stereoelectronic effect is also used to emphasize the relation between the electronic structure and the geometry (stereochemistry) of a molecule.
The Yukawa–Tsuno equation, first developed in 1959, is a linear free-energy relationship in physical organic chemistry. It is a modified version of the Hammett equation that accounts for enhanced resonance effects in electrophilic reactions of para- and meta-substituted organic compounds. This equation does so by introducing a new term to the original Hammett relation that provides a measure of the extent of resonance stabilization for a reactive structure that builds up charge in its transition state. The Yukawa–Tsuno equation can take the following forms:
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction.
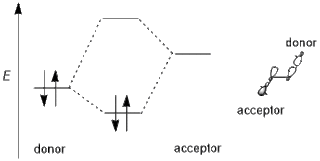
In chemistry, primarily organic and computational chemistry, a stereoelectronic effect is an effect on molecular geometry, reactivity, or physical properties due to spatial relationships in the molecules' electronic structure, in particular the interaction between atomic and/or molecular orbitals. Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the ground and/or transition states of molecules that arise from considerations of orbital overlap. Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientations of electrons in space.
In organic chemistry, the Cieplak effect is a predictive model to rationalize why nucleophiles preferentially add to one face of a carbonyl over another. Proposed by Andrzej Stanislaw Cieplak in 1980, it correctly predicts results that could not be justified by the other standard models at the time, such as the Cram and Felkin–Anh models. In the Cieplak model, electrons from a neighboring bond delocalize into the forming carbon–nucleophile (C–Nuc) bond, lowering the energy of the transition state and accelerating the rate of reaction. Whichever bond can best donate its electrons into the C–Nuc bond determines which face of the carbonyl the nucleophile will add to. The nucleophile may be any of a number of reagents, most commonly organometallic or reducing agents. The Cieplak effect is subtle, and often competes with sterics, solvent effects, counterion complexation of the carbonyl oxygen, and other effects to determine product distribution. Subsequent work has questioned its legitimacy.

In chemistry, pi stacking refers to the presumptive attractive, noncovalent pi interactions between the pi bonds of aromatic rings. According to some authors direct stacking of aromatic rings is electrostatically repulsive.
The pEDA parameter is a pi-electron substituent effect scale, described also as mesomeric or resonance effect. There is also a complementary scale - sEDA. The more positive is the value of pEDA the more pi-electron donating is a substituent. The more negative pEDA, the more pi-electron withdrawing is the substituent.
References
- ↑ Ozimiński, Wojciech P.; Dobrowolski, Jan C. (2009-08-01). "σ- and π-electron contributions to the substituent effect: natural population analysis". Journal of Physical Organic Chemistry. 22 (8): 769–778. doi:10.1002/poc.1530. ISSN 1099-1395.
- ↑ Boyd, Russell J.; Edgecombe, Kenneth E. (1988-06-01). "Atomic and group electronegativities from the electron-density distributions of molecules". Journal of the American Chemical Society. 110 (13): 4182–4186. doi:10.1021/ja00221a014. ISSN 0002-7863.