Related Research Articles
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.

Fiber is a natural or artificial substance that is significantly longer than it is wide. Fibers are often used in the manufacture of other materials. The strongest engineering materials often incorporate fibers, for example carbon fiber and ultra-high-molecular-weight polyethylene.
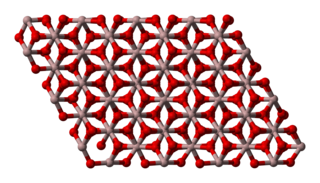
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Glass fiber is a material consisting of numerous extremely fine fibers of glass.
Fiberglass or fibreglass is a common type of fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened into a sheet called a chopped strand mat, or woven into glass cloth. The plastic matrix may be a thermoset polymer matrix—most often based on thermosetting polymers such as epoxy, polyester resin, or vinyl ester resin—or a thermoplastic.

Mineral wool is any fibrous material formed by spinning or drawing molten mineral or rock materials such as slag and ceramics.
A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. All of the period 3 elements occur in nature and have at least one stable isotope.

Pulp is a fibrous lignocellulosic material prepared by chemically, semi-chemically or mechanically producing cellulosic fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemicals or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products.

Silica gel is an amorphous and porous form of silicon dioxide (silica), consisting of an irregular tridimensional framework of alternating silicon and oxygen atoms with nanometer-scale voids and pores. The voids may contain water or some other liquids, or may be filled by gas or vacuum. In the last case, the material is properly called silica xerogel.
Sodium silicate is a generic name for chemical compounds with the formula Na
2xSi
yO
2y+x or (Na
2O)
x·(SiO
2)
y, such as sodium metasilicate, sodium orthosilicate, and sodium pyrosilicate. The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts.
The Bayer process is the principal industrial means of refining bauxite to produce alumina (aluminium oxide) and was developed by Carl Josef Bayer. Bauxite, the most important ore of aluminium, contains only 30–60% aluminium oxide (Al2O3), the rest being a mixture of silica, various iron oxides, and titanium dioxide. The aluminium oxide must be further purified before it can be refined into aluminium.

Polyacrylonitrile (PAN) is a synthetic, semicrystalline organic polymer resin, with the linear formula (CH2CHCN)n. Almost all PAN resins are copolymers with acrylonitrile as the main monomer. PAN is used to produce large variety of products including ultra filtration membranes, hollow fibers for reverse osmosis, fibers for textiles, and oxidized PAN fibers. PAN fibers are the chemical precursor of very high-quality carbon fiber. PAN is first thermally oxidized in air at 230 °C to form an oxidized PAN fiber and then carbonized above 1000 °C in inert atmosphere to make carbon fibers found in a variety of both high-tech and common daily applications such as civil and military aircraft primary and secondary structures, missiles, solid propellant rocket motors, pressure vessels, fishing rods, tennis rackets and bicycle frames. It is a component repeat unit in several important copolymers, such as styrene-acrylonitrile (SAN) and acrylonitrile butadiene styrene (ABS) plastic.

Soda lime, a mixture of sodium hydroxide (NaOH) and calcium oxide (CaO), is used in granular form within recirculating breathing environments like general anesthesia and its breathing circuit, submarines, rebreathers, and hyperbaric chambers and underwater habitats. Its purpose is to eliminate carbon dioxide from breathing gases, preventing carbon dioxide retention and, eventually, carbon dioxide poisoning. The creation of soda lime involves treating slaked lime with a concentrated sodium hydroxide solution.

In geology, petrifaction or petrification is the process by which organic material becomes a fossil through the replacement of the original material and the filling of the original pore spaces with minerals. Petrified wood typifies this process, but all organisms, from bacteria to vertebrates, can become petrified. Petrification takes place through a combination of two similar processes: permineralization and replacement. These processes create replicas of the original specimen that are similar down to the microscopic level.

In materials science, a refractory is a material that is resistant to decomposition by heat or chemical attack and that retains its strength and rigidity at high temperatures. They are inorganic, non-metallic compounds that may be porous or non-porous, and their crystallinity varies widely: they may be crystalline, polycrystalline, amorphous, or composite. They are typically composed of oxides, carbides or nitrides of the following elements: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. Many refractories are ceramics, but some such as graphite are not, and some ceramics such as clay pottery are not considered refractory. Refractories are distinguished from the refractory metals, which are elemental metals and their alloys that have high melting temperatures.
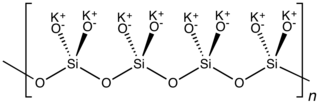
Potassium silicate is the name for a family of inorganic compounds. The most common potassium silicate has the formula K2SiO3, samples of which contain varying amounts of water. These are white solids or colorless solutions.
A geopolymer is a vague pseudo-chemical term used to describe inorganic, typically bulk ceramic-like material that forms covalently bonded, non-crystalline (amorphous) networks, often intermingled with other phases. Many geopolymers may also be classified as alkali-activated cements or acid-activated binders. They are mainly produced by a chemical reaction between a chemically reactive aluminosilicate powder e.g. metakaolin or other clay-derived powders, natural pozzolan, or suitable glasses, and an aqueous solution that causes this powder to react and re-form into a solid monolith. The most common pathway to produce geopolymers is by the reaction of metakaolin with sodium silicate, which is an alkaline solution, but other processes are also possible.
Dealkalization is a process of surface modification applicable to glasses containing alkali ions, wherein a thin surface layer is created that has a lower concentration of alkali ions than is present in the underlying, bulk glass. This change in surface composition commonly alters the observed properties of the surface, most notably enhancing corrosion resistance.
Deinking is the industrial process of removing printing ink from paperfibers of recycled paper to make deinked pulp.
Porous glass is glass that includes pores, usually in the nanometre- or micrometre-range, commonly prepared by one of the following processes: through metastable phase separation in borosilicate glasses (such as in their system SiO2-B2O3-Na2O), followed by liquid extraction of one of the formed phases; through the sol-gel process; or simply by sintering glass powder.
References
- ↑ Canadian Patent: CA 1142312, Application Number: 343548 Inventors: Hillermeier, Karlheinz (Germany), Seeberger, Ernst (Germany) Owners: AKZO N.V. (Netherlands)
- Water-Containing Water Glass Fibers: United States Patent: 4,471,019, Sep. 11, 1984
- Manufacturing Silica Fibers. United States Patent, 4,332,601, Jun. 1, 1982. Arno Wegerhoff, Woerth and Karlheinz Hillermeier, Wuppertal. All of Fed. Rep. of Germany