
Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g. "glass", "glasses", "magnifying glass".

Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored.

Fiber is a natural or artificial substance that is significantly longer than it is wide. Fibers are often used in the manufacture of other materials. The strongest engineering materials often incorporate fibers, for example carbon fiber and ultra-high-molecular-weight polyethylene.
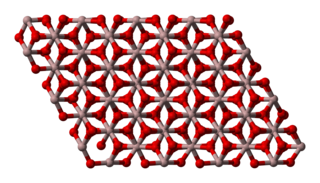
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Glass fiber is a material consisting of numerous extremely fine fibers of glass.
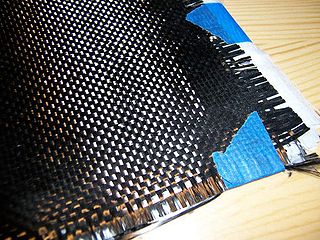
Carbon fibers or carbon fibres are fibers about 5 to 10 micrometers (0.00020–0.00039 in) in diameter and composed mostly of carbon atoms. Carbon fibers have several advantages: high stiffness, high tensile strength, high strength to weight ratio, high chemical resistance, high-temperature tolerance, and low thermal expansion. These properties have made carbon fiber very popular in aerospace, civil engineering, military, motorsports, and other competition sports. However, they are relatively expensive compared to similar fibers, such as glass fiber, basalt fibers, or plastic fibers.
Fiberglass or fibreglass is a common type of fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened into a sheet called a chopped strand mat, or woven into glass cloth. The plastic matrix may be a thermoset polymer matrix—most often based on thermosetting polymers such as epoxy, polyester resin, or vinyl ester resin—or a thermoplastic.

Silicon carbide (SiC), also known as carborundum, is a hard chemical compound containing silicon and carbon. A wide bandgap semiconductor, it occurs in nature as the extremely rare mineral moissanite, but has been mass-produced as a powder and crystal since 1893 for use as an abrasive. Grains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in applications requiring high endurance, such as car brakes, car clutches and ceramic plates in bulletproof vests. Large single crystals of silicon carbide can be grown by the Lely method and they can be cut into gems known as synthetic moissanite.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.

Fused quartz, fused silica or quartz glass is a glass consisting of almost pure silica (silicon dioxide, SiO2) in amorphous (non-crystalline) form. This differs from all other commercial glasses, such as soda-lime glass, lead glass, or borosilicate glass, in which other ingredients are added which change the glasses' optical and physical properties, such as lowering the melt temperature, the spectral transmission range, or the mechanical strength. Fused quartz, therefore, has high working and melting temperatures, making it difficult to form and less desirable for most common applications, but is much stronger, more chemically resistant, and exhibits lower thermal expansion, making it more suitable for many specialized uses such as lighting and scientific applications.
Fibre-reinforced plastic is a composite material made of a polymer matrix reinforced with fibres. The fibres are usually glass, carbon, aramid, or basalt. Rarely, other fibres such as paper, wood, boron, or asbestos have been used. The polymer is usually an epoxy, vinyl ester, or polyester thermosetting plastic, though phenol formaldehyde resins are still in use.

In the textile industry, a tow is a coarse, broken fibre, removed during processing flax, hemp, or jute and separated from the shives. Flax tows are often used as upholstery stuffing and oakum. Tows in general are frequently cut up to produce staple fibre. The very light color of flax tow is the source of the word "towhead", meaning a person with naturally light blond hair.

Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion, making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about 165 °C (300 °F). It is commonly used for the construction of reagent bottles and flasks, as well as lighting, electronics, and cookware. For many other applications, soda-lime glass is more common.
Basalt fibers are produced from basalt rocks by melting them and converting the melt into fibers. Basalts are rocks of igneous origin. The main energy consumption for the preparation of basalt raw materials to produce of fibers is made in natural conditions. Basalt fibers are classified into 3 types:
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.
Bulk moulding compound (BMC), bulk moulding composite, or dough moulding compound (DMC), is a ready-to-mold, glass-fiber reinforced thermoset polymer material primarily used in compression moulding, as well as in injection moulding and transfer moulding. Typical applications include demanding electrical applications, corrosion resistant needs, appliance, automotive, and transit.

Filler materials are particles added to resin or binders that can improve specific properties, make the product cheaper, or a mixture of both. The two largest segments for filler material use is elastomers and plastics. Worldwide, more than 53 million tons of fillers are used every year in application areas such as paper, plastics, rubber, paints, coatings, adhesives, and sealants. As such, fillers, produced by more than 700 companies, rank among the world's major raw materials and are contained in a variety of goods for daily consumer needs. The top filler materials used are ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), kaolin, talc, and carbon black.
Polymer engineering is generally an engineering field that designs, analyses, and modifies polymer materials. Polymer engineering covers aspects of the petrochemical industry, polymerization, structure and characterization of polymers, properties of polymers, compounding and processing of polymers and description of major polymers, structure property relations and applications.
Carbon fiber-reinforced polymers, carbon-fibre-reinforced polymers, carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic, also known as carbon fiber, carbon composite, or just carbon, are extremely strong and light fiber-reinforced plastics that contain carbon fibers. CFRPs can be expensive to produce, but are commonly used wherever high strength-to-weight ratio and stiffness (rigidity) are required, such as aerospace, superstructures of ships, automotive, civil engineering, sports equipment, and an increasing number of consumer and technical applications.
Glass typically has a tensile strength of 7 megapascals (1,000 psi). However, the theoretical upper bound on its strength is orders of magnitude higher: 17 gigapascals (2,500,000 psi). This high value is due to the strong chemical Si–O bonds of silicon dioxide. Imperfections of the glass, such as bubbles, and in particular surface flaws, such as scratches, have a great effect on the strength of glass and decrease it even more than for other brittle materials. The chemical composition of the glass also impacts its tensile strength. The processes of thermal and chemical toughening can increase the tensile strength of glass.











