Related Research Articles

Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy, and solar architecture. It is an essential source of renewable energy, and its technologies are broadly characterized as either passive solar or active solar depending on how they capture and distribute solar energy or convert it into solar power. Active solar techniques include the use of photovoltaic systems, concentrated solar power, and solar water heating to harness the energy. Passive solar techniques include orienting a building to the Sun, selecting materials with favorable thermal mass or light-dispersing properties, and designing spaces that naturally circulate air.
In photochemistry, photohydrogen is hydrogen produced with the help of artificial or natural light. This is how the leaf of a tree splits water molecules into protons, electrons and oxygen. Photohydrogen may also be produced by the photodissociation of water by ultraviolet light.

Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors.
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water.
Artificial photosynthesis is a chemical process that biomimics the natural process of photosynthesis. The term artificial photosynthesis is used loosely, refer to any scheme for capturing and storing energy from sunlight by producing a fuel, specifically a solar fuel. An advantage of artificial photosynthesis is that the solar energy can be immediately converted and stored. By contrast, using photovoltaic cells, sunlight is converted into electricity and then converted again into chemical energy for storage, with some necessary losses of energy associated with the second conversion. The byproducts of these reactions are environmentally friendly. Artificially photosynthesized fuel would be a carbon-neutral source of energy, which could be used for transportation or homes. The economics of artificial photosynthesis are not competitive.

Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
In the 19th century, it was observed that the sunlight striking certain materials generates detectable electric current – the photoelectric effect. This discovery laid the foundation for solar cells. Solar cells have gone on to be used in many applications. They have historically been used in situations where electrical power from the grid was unavailable.

Electrolysis of water is using electricity to split water into oxygen and hydrogen gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture would be extremely explosive. Separately pressurised into convenient 'tanks' or 'gas bottles', hydrogen can be used for oxyhydrogen welding and other applications, as the hydrogen / oxygen flame can reach approximately 2,800°C.
Hydrogen gas is produced by several industrial methods. In 2022 less than 1% of hydrogen production was low-carbon. Fossil fuels are the dominant source of hydrogen, for example by steam reforming of natural gas. Other methods of hydrogen production include biomass gasification and methane pyrolysis. Methane pyrolysis and water electrolysis can use any source of electricity including renewable energy. Underground hydrogen is extracted.

Solar power, also known as solar electricity, is the conversion of energy from sunlight into electricity, either directly using photovoltaics (PV) or indirectly using concentrated solar power. Solar panels use the photovoltaic effect to convert light into an electric current. Concentrated solar power systems use lenses or mirrors and solar tracking systems to focus a large area of sunlight to a hot spot, often to drive a steam turbine.
Photoelectrochemistry is a subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems. It is an active domain of investigation. One of the pioneers of this field of electrochemistry was the German electrochemist Heinz Gerischer. The interest in this domain is high in the context of development of renewable energy conversion and storage technology.
A solar fuel is a synthetic chemical fuel produced from solar energy. Solar fuels can be produced through photochemical, photobiological, and electrochemical reactions.
The following outline is provided as an overview of and topical guide to solar energy:
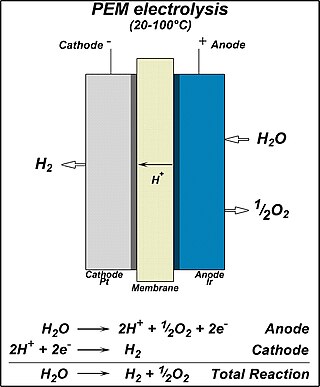
Proton exchange membrane(PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the electrodes. The PEM electrolyzer was introduced to overcome the issues of partial load, low current density, and low pressure operation currently plaguing the alkaline electrolyzer. It involves a proton-exchange membrane.
Biological photovoltaics, also called biophotovoltaics or BPV, is an energy-generating technology which uses oxygenic photoautotrophic organisms, or fractions thereof, to harvest light energy and produce electrical power. Biological photovoltaic devices are a type of biological electrochemical system, or microbial fuel cell, and are sometimes also called photo-microbial fuel cells or “living solar cells”. In a biological photovoltaic system, electrons generated by photolysis of water are transferred to an anode. A relatively high-potential reaction takes place at the cathode, and the resulting potential difference drives current through an external circuit to do useful work. It is hoped that using a living organism as the light harvesting material, will make biological photovoltaics a cost-effective alternative to synthetic light-energy-transduction technologies such as silicon-based photovoltaics.
Hydrogenics is a developer and manufacturer of hydrogen generation and fuel cell products based on water electrolysis and proton-exchange membrane (PEM) technology. Hydrogenics is divided into two business units: OnSite Generation and Power Systems. Onsite Generation is headquartered in Oevel, Belgium and had 73 full-time employees as of December 2013. Power Systems is based in Mississauga, Ontario, Canada, with a satellite facility in Gladbeck, Germany. It had 62 full-time employees as of December 2013. Hydrogenics maintains operations in Belgium, Canada and Germany with satellite offices in the United States, Indonesia, Malaysia and Russia.
The Bionic Leaf is a biomimetic system that gathers solar energy via photovoltaic cells that can be stored or used in a number of different functions. Bionic leaves can be composed of both synthetic and organic materials (bacteria), or solely made of synthetic materials. The Bionic Leaf has the potential to be implemented in communities, such as urbanized areas to provide clean air as well as providing needed clean energy.

Kevin Sivula is a highly cited American chemical engineer and researcher in the field of solar cells. He is a professor of molecular engineering at EPFL and the head of the Laboratory for Molecular Engineering of Optoelectronic Nanomaterials at EPFL's School of Basic Sciences.

Emily Warren is an American chemical engineer who is a staff scientist at the National Renewable Energy Laboratory. Her research considers high efficiency crystalline photovoltaics.
Solar reforming is the sunlight-driven conversion of diverse carbon waste resources into sustainable fuels and value-added chemicals. It encompasses a set of technologies operating under ambient and aqueous conditions, utilizing solar spectrum to generate maximum value. Solar reforming offers an attractive and unifying solution to address the contemporary challenges of climate change and environmental pollution by creating a sustainable circular network of waste upcycling, clean fuel generation and the consequent mitigation of greenhouse emissions.
References
- 1 2 3 "Schatz Solar Hydrogen Project". schatzlab.org. Retrieved 2011-06-18.
- ↑ Nakato, Y.; Jia, G.; Ishida, M.; Morisawa, K.; Fujitani, M.; Hinogami, R.; Yae, S. (10 June 1998). "Efficient Solar-to-Chemical Conversion by One Chip of n-Type Silicon with Surface Asymmetry". Electrochem. Solid-State Lett. 1 (2). Osaka, Japan: Electrochemical Society: 71–73. doi:10.1149/1.1390640 . Retrieved 2011-07-20.
- ↑ Yae, Shinji; Kobayashi, Tsutomu; Abe, Makoto; Nasu, Noriaki; Fukumuro, Naoki; Ogawa, Shunsuke; Yoshida, Norimitsu; Nonomura, Shuichi; Nakato, Yoshihiro; Matsuda, Hitoshi (15 February 2007). "Solar to chemical conversion using metal nanoparticle modified microcrystalline silicon thin film photoelectrode". Solar Energy Materials and Solar Cells. 91 (4). Japan: ScienceDirect: 224–229. doi:10.1016/j.solmat.2006.08.010.
- 1 2 3 "Water splitting to produce solar hydrogen using silicon thin film". spie.org. Retrieved 2011-08-30.
- ↑ Fujishima, Akira; Honda, Kenichi (7 July 1972). "Electrochemical Photolysis of Water at a Semiconductor Electrode". Nature . 238 (1). Japan: Nature Publishing Group: 37–38. doi:10.1038/238037a0. PMID 12635268.