Soluble transferrin receptor conventionally refers to the cleaved extracellular portion of transferrin receptor 1 that is released into serum. This receptor is a protein dimer of two identical subunits, linked together by two pairs of disulfide bonds. Its molecular mass 190,000 Dalton. [1]
Blood testing of the soluble transferrin receptor (sTfR) is used as a measure of functional iron status and the investigation of iron deficiency anemia. Ferritin, a routine investigation for anemia, is an acute-phase reactant, and may be elevated in states of inflammation, thereby falsely indicating that iron stores are adequate. [2] Because sTfR is insensitive to inflammation, it can detect anemia in patients with preexisting inflammatory states, and is particularly useful in distinguishing between the anemia of chronic disease and anemias caused by lack of iron intake. [3]
To date, the conventionally identified soluble transferrin receptor has not been itself implicated in intracellular delivery of transferrin and associated iron stores.
A soluble receptor for any ligand could also refer to a molecule present is solution (for example a secretory protein) which would bind with the target ligand and then effect cellular delivery. In this context the multifunctional glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has been reported as a soluble receptor for transferrin. It has been demonstrated to deliver more transferrin as compared to the receptors anchored on the cells surface in numerous cell types. [4]

Iron deficiency, or sideropenia, is the state in which a body lacks enough iron to supply its needs. Iron is present in all cells in the human body and has several vital functions, such as carrying oxygen to the tissues from the lungs as a key component of the hemoglobin protein, acting as a transport medium for electrons within the cells in the form of cytochromes, and facilitating oxygen enzyme reactions in various tissues. Too little iron can interfere with these vital functions and lead to morbidity and death.

Ferritin is a universal intracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. It is the primary intracellular iron-storage protein in both prokaryotes and eukaryotes, keeping iron in a soluble and non-toxic form. In humans, it acts as a buffer against iron deficiency and iron overload.
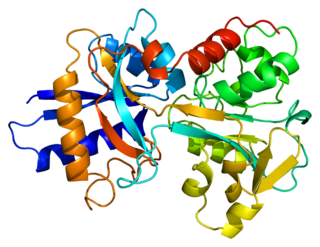
Transferrins are glycoproteins found in vertebrates which bind and consequently mediate the transport of iron (Fe) through blood plasma. They are produced in the liver and contain binding sites for two Fe3+ ions. Human transferrin is encoded by the TF gene and produced as a 76 kDa glycoprotein.

Acute-phase proteins (APPs) are a class of proteins whose concentrations in blood plasma either increase or decrease in response to inflammation. This response is called the acute-phase reaction. The acute-phase reaction characteristically involves fever, acceleration of peripheral leukocytes, circulating neutrophils and their precursors. The terms acute-phase protein and acute-phase reactant (APR) are often used synonymously, although some APRs are polypeptides rather than proteins.
Iron-deficiency anemia is anemia caused by a lack of iron. Anemia is defined as a decrease in the number of red blood cells or the amount of hemoglobin in the blood. When onset is slow, symptoms are often vague such as feeling tired, weak, short of breath, or having decreased ability to exercise. Anemia that comes on quickly often has more severe symptoms, including confusion, feeling like one is going to pass out or increased thirst. Anemia is typically significant before a person becomes noticeably pale. Children with iron deficiency anemia may have problems with growth and development. There may be additional symptoms depending on the underlying cause.
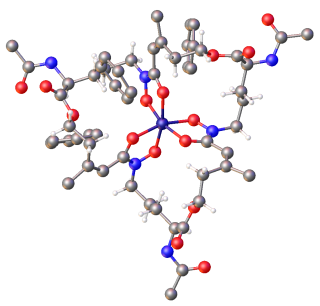
Siderophores (Greek: "iron carrier") are small, high-affinity iron-chelating compounds that are secreted by microorganisms such as bacteria and fungi. They help the organism accumulate iron. Although a widening range of siderophore functions is now being appreciated, siderophores are among the strongest (highest affinity) Fe3+ binding agents known. Phytosiderophores are siderophores produced by plants.
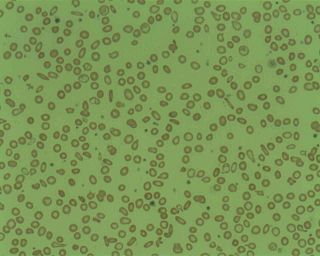
Microcytic anaemia is any of several types of anemia characterized by smaller than normal red blood cells. The normal mean corpuscular volume is approximately 80–100 fL. When the MCV is <80 fL, the red cells are described as microcytic and when >100 fL, macrocytic. The MCV is the average red blood cell size.
Anemia of chronic disease (ACD) or anemia of chronic inflammation is a form of anemia seen in chronic infection, chronic immune activation, and malignancy. These conditions all produce elevation of interleukin-6, which stimulates hepcidin production and release from the liver. Hepcidin production and release shuts down ferroportin, a protein that controls export of iron from the gut and from iron storing cells. As a consequence, circulating iron levels are reduced. Other mechanisms may also play a role, such as reduced erythropoiesis. It is also known as anemia of inflammation, or anemia of inflammatory response.
Serum iron is a medical laboratory test that measures the amount of circulating iron that is bound to transferrin and freely circulate in the blood. Clinicians order this laboratory test when they are concerned about iron deficiency, which can cause anemia and other problems. 65% of the iron in the body is bound up in hemoglobin molecules in red blood cells. About 4% is bound up in myoglobin molecules. Around 30% of the iron in the body is stored as ferritin or hemosiderin in the spleen, the bone marrow and the liver. Small amounts of iron can be found in other molecules in cells throughout the body. None of this iron is directly accessible by testing the serum.}

Total iron-binding capacity (TIBC) or sometimes transferrin iron-binding capacity is a medical laboratory test that measures the blood's capacity to bind iron with transferrin. Transferrin can bind two atoms of ferric iron (Fe3+) with high affinity. It means that transferrin has the capacity to transport approximately from 1.40 to 1.49 mg of iron per gram of transferrin present in the blood.

Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron at the systemic and cellular level. Iron is both necessary to the body and potentially toxic. Controlling iron levels in the body is a critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism, because iron is essential for red blood cells, where most of the human body's iron is contained. Understanding iron metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron-deficiency anemia.
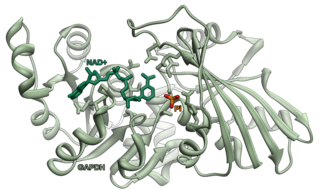
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme of about 37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules. In addition to this long established metabolic function, GAPDH has recently been implicated in several non-metabolic processes, including transcription activation, initiation of apoptosis, ER to Golgi vesicle shuttling, and fast axonal, or axoplasmic transport. In sperm, a testis-specific isoenzyme GAPDHS is expressed.
Iron-binding proteins are carrier proteins and metalloproteins that are important in iron metabolism and the immune response. Iron is required for life.

Atransferrinemia is an autosomal recessive metabolic disorder in which there is an absence of transferrin, a plasma protein that transports iron through the blood. Atransferrinemia is characterized by anemia and hemosiderosis in the heart and liver. The iron damage to the heart can lead to heart failure. The anemia is typically microcytic and hypochromic. Atransferrinemia was first described in 1961 and is extremely rare, with only ten documented cases worldwide.

Transferrin receptor (TfR) is a carrier protein for transferrin. It is needed for the import of iron into cells and is regulated in response to intracellular iron concentration. It imports iron by internalizing the transferrin-iron complex through receptor-mediated endocytosis. The existence of a receptor for transferrin iron uptake has been recognized since the late 1950s. Earlier two transferrin receptors in humans, transferrin receptor 1 and transferrin receptor 2 had been characterized and until recently cellular iron uptake was believed to occur chiefly via these two well documented transferrin receptors. Both these receptors are transmembrane glycoproteins. TfR1 is a high affinity ubiquitously expressed receptor while expression of TfR2 is restricted to certain cell types and is unaffected by intracellular iron concentrations. TfR2 binds to transferrin with a 25-30 fold lower affinity than TfR1. Although TfR1 mediated iron uptake is the major pathway for iron acquisition by most cells and especially developing erythrocytes, several studies have indicated that the uptake mechanism varies depending upon the cell type. It is also reported that Tf uptake exists independent of these TfRs although the mechanisms are not well characterized. The multifunctional glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase has been shown to utilize post translational modifications to exhibit higher order moonlighting behavior wherein it switches its function as a holo or apo transferrin receptor leading to either iron delivery or iron export respectively.

Iron is an important biological element. It is used in both the ubiquitous Iron-sulfur proteins and in Vertebrates it is used in Hemoglobin which is essential for Blood and oxygen transport.
Sideropenic hypochromic anemia is primarily characterized by low serum iron concentration. Non-sideropenic hypochromic anemia is the ineffective utilisation of iron stores, usually from chronic infection/inflammation. It is very important to distinguish iron deficit anemia from the anemia of chronic disorders so as to ensure specific treatment.

Protein moonlighting is a phenomenon by which a protein can perform more than one function. It is an excellent example of gene sharing.
Latent iron deficiency (LID), also called iron-deficient erythropoiesis, is a medical condition in which there is evidence of iron deficiency without anemia. It is important to assess this condition because individuals with latent iron deficiency may develop iron-deficiency anemia. Additionally, there is some evidence of a decrease in vitality and an increase in fatigue among individuals with LID.
Iron preparation is the formulation for iron supplements indicated in prophylaxis and treatment of iron-deficiency anemia. Examples of iron preparation include ferrous sulfate, ferrous gluconate, and ferrous fumarate. It can be administered orally, and by intravenous injection, or intramuscular injection.













