The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.
The Therapeutic Goods Administration (TGA) is the medicine and therapeutic regulatory agency of the Australian Government. As part of the Department of Health and Aged Care, the TGA regulates the quality, supply and advertising of medicines, pathology devices, medical devices, blood products and most other therapeutics. Any items that claim to have a therapeutic effect, are involved in the administration of medication, or are otherwise covered by the Therapeutic Goods Act 1989, the Therapeutic Goods Regulations 1990, or a ministerial order, must be approved by the TGA and registered in the Australian Register of Therapeutic Goods.

The Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for prevention of COVID-19. Developed in the United Kingdom by Oxford University and British-Swedish company AstraZeneca, using as a vector the modified chimpanzee adenovirus ChAdOx1. The vaccine is given by intramuscular injection. Studies carried out in 2020 showed that the efficacy of the vaccine is 76.0% at preventing symptomatic COVID-19 beginning at 22 days following the first dose, and 81.3% after the second dose. A study in Scotland found that, for symptomatic COVID-19 infection after the second dose, the vaccine is 81% effective against the Alpha variant, and 61% against the Delta variant.

COVID-19 Vaccines Global Access, abbreviated as COVAX, is a worldwide initiative aimed at equitable access to COVID-19 vaccines directed by the GAVI vaccine alliance, the Coalition for Epidemic Preparedness Innovations (CEPI), and the World Health Organization (WHO), alongside key delivery partner UNICEF. It is one of the four pillars of the Access to COVID-19 Tools Accelerator, an initiative begun in April 2020 by the WHO, the European Commission, and the government of France as a response to the COVID-19 pandemic. COVAX coordinates international resources to enable low-to-middle-income countries equitable access to COVID-19 tests, therapies, and vaccines. UNICEF is the key delivery partner, leveraging its experience as the largest single vaccine buyer in the world and working on the procurement of COVID-19 vaccine doses, as well as logistics, country readiness and in-country delivery.

The COVID-19 vaccination programme in the United Kingdom is an ongoing mass immunisation campaign for coronavirus disease 2019 (COVID-19) during the COVID-19 pandemic in the United Kingdom.
The COVID-19 vaccination program in the Philippines is an ongoing mass immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country. The vaccination program was initiated by the Duterte administration on March 1, 2021, a day after the arrival of the country's first vaccine doses which were donated by the Chinese government.

The COVID-19 vaccination campaign in Italy is a mass immunization campaign that was put in place by the Italian government in order to respond to the ongoing COVID-19 pandemic. It started on 27 December 2020, together with most countries in the European Union.

SARS-CoV-2, the virus that causes COVID-19, was isolated in late 2019. Its genetic sequence was published on 11 January 2020, triggering an urgent international response to prepare for an outbreak and hasten the development of a preventive COVID-19 vaccine. Since 2020, vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. By June 2020, tens of billions of dollars were invested by corporations, governments, international health organizations, and university research groups to develop dozens of vaccine candidates and prepare for global vaccination programs to immunize against COVID‑19 infection. According to the Coalition for Epidemic Preparedness Innovations (CEPI), the geographic distribution of COVID‑19 vaccine development shows North American entities to have about 40% of the activity, compared to 30% in Asia and Australia, 26% in Europe, and a few projects in South America and Africa.

The general COVID-19 vaccination in Australia program began on 22 February 2021 in response to the COVID-19 pandemic, with the goal of vaccinating all willing people in Australia before 2022. Front-line workers and aged care staff and residents had priority for being inoculated, before a gradual phased release to less-vulnerable and lower-risk population groups throughout 2021. The Therapeutic Goods Administration (TGA) approved four vaccines for Australian use in 2021: the Pfizer–BioNTech vaccine on 25 January, the Oxford–AstraZeneca vaccine on 16 February, Janssen vaccine on 25 June and the Moderna vaccine on 9 August. Although approved for use, the Janssen vaccine was not included in the Australian vaccination program as of June 2021.

A dispute broke out in January 2021 between the European Commission and the pharmaceutical company AstraZeneca AB about the provision of COVID-19 vaccines during the COVID-19 pandemic, and, in February, spilled out into a dispute over Article 16 of the Northern Ireland Protocol. Vaccination proceeded apace in the UK but more slowly in the EU, and by the end of March 2021, over 30% of the UK population had received at least one dose of vaccine compared to about 8% of the EU population. This was partly due to limited availability of the AstraZeneca vaccine in the EU. The World Health Organization and the European Medicines Agency continued to state that the vaccine was safe and effective. However, a representative of the European Medicines Agency said in June that vaccines based on the mRNA technology should be preferred if available for all age groups, including for the over 60s.

The COVID-19 vaccination programme in the Republic of Ireland is an ongoing mass immunisation campaign that began on 29 December 2020 in response to the COVID-19 pandemic in the Republic of Ireland. Ireland's vaccination rollout has been praised as one of the most successful rollouts in the world and was ranked number one in the European Union in terms of its percentage of adult population fully vaccinated, and was also ranked number one in the EU for the number of booster vaccines administered.

COVID-19 vaccination in Canada is an ongoing, intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic in Canada. Provinces have worked with local municipal governments, hospital systems, family doctors and independently owned pharmacies to aid in part, or in full with vaccination rollout. The vaccination effort in full is the largest such immunization effort in the nation's history. The vaccination effort began December 14, 2020, and is currently ongoing.

COVID-19 vaccination in South Africa is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
COVID-19 vaccination in Botswana is an ongoing immunisation campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.
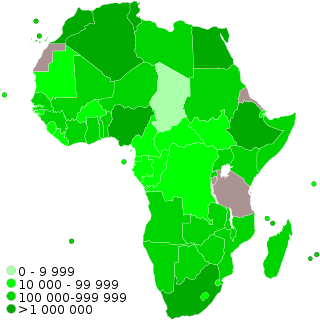
COVID-19 vaccination programs have begun in many countries and territories in Africa. In June 2021, the World Health Organization predicted that 47 of Africa's 54 nations would fall short of the aim of vaccinating 10% of their people by September 2021. In June, Africa accounted for fewer than 1% of worldwide vaccine doses delivered. Africa received in total less than 2% of the 3 billion vaccination doses provided globally.

Post-vaccination embolic and thrombotic events, termed vaccine-induced immune thrombotic thrombocytopenia (VITT), vaccine-induced prothrombotic immune thrombocytopenia (VIPIT), thrombosis with thrombocytopenia syndrome (TTS), vaccine-induced immune thrombocytopenia and thrombosis (VITT), or vaccine-associated thrombotic thrombocytopenia (VATT), are rare types of blood clotting syndromes that were initially observed in a number of people who had previously received the Oxford–AstraZeneca COVID‑19 vaccine (AZD1222) during the COVID‑19 pandemic. It was subsequently also described in the Janssen COVID‑19 vaccine, leading to the suspension of its use until its safety had been reassessed. On 5 May 2022 the FDA posted a bulletin limiting the use of the Janssen Vaccine to very specific cases due to further reassessment of the risks of TTS, although the FDA also stated in the same bulletin that the benefits of the vaccine outweigh the risks.

COVID-19 vaccination in South Korea is an ongoing immunization campaign against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), in response to the ongoing pandemic in the country.

The COVID-19 vaccination in Indonesia is an ongoing mass immunization in response to the COVID-19 pandemic in Indonesia. On 13 January 2021, the program commenced when President Joko Widodo was vaccinated at the presidential palace. In terms of total doses given, Indonesia ranks third in Asia and fifth in the world.
COVID-19 vaccination in Ontario began in December 2020, when the first doses of the Pfizer vaccine were administered. In February 2021, shipments for both the Pfizer and Moderna vaccines increased significantly. By May 2021, over 50 percent of Ontarians had received their first dose. By the beginning of 2022, over 80 percent of Ontarians had received their first dose.













