Related Research Articles

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.
Hans Spemann was a German embryologist who was awarded a Nobel Prize in Physiology or Medicine in 1935 for his student Hilde Mangold's discovery of the effect now known as embryonic induction, an influence, exercised by various parts of the embryo, that directs the development of groups of cells into particular tissues and organs. Spemann added his name as an author to Hilde Mangold's dissertation and won a Nobel Prize for her work.

Neurulation refers to the folding process in vertebrate embryos, which includes the transformation of the neural plate into the neural tube. The embryo at this stage is termed the neurula.
Organogenesis is the phase of embryonic development that starts at the end of gastrulation and continues until birth. During organogenesis, the three germ layers formed from gastrulation form the internal organs of the organism.
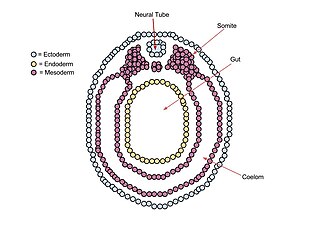
A neurula is a vertebrate embryo at the early stage of development in which neurulation occurs. The neurula stage is preceded by the gastrula stage; consequentially, neurulation is preceded by gastrulation. Neurulation marks the beginning of the process of organogenesis.
The primitive node is the organizer for gastrulation in most amniote embryos. In birds, it is known as Hensen's node, and in amphibians, it is known as the Spemann-Mangold organizer. It is induced by the Nieuwkoop center in amphibians, or by the posterior marginal zone in amniotes including birds.

The primitive streak is a structure that forms in the early embryo in amniotes. In amphibians, the equivalent structure is the blastopore. During early embryonic development, the embryonic disc becomes oval shaped, and then pear-shaped with the broad end towards the anterior, and the narrower region projected to the posterior. The primitive streak forms a longitudinal midline structure in the narrower posterior (caudal) region of the developing embryo on its dorsal side. At first formation, the primitive streak extends for half the length of the embryo. In the human embryo, this appears by stage 6, about 17 days.

In amniote embryonic development, the epiblast is one of two distinct cell layers arising from the inner cell mass in the mammalian blastocyst, or from the blastula in reptiles and birds, the other layer is the hypoblast. It drives the embryo proper through its differentiation into the three primary germ layers, ectoderm, mesoderm and endoderm, during gastrulation. The amniotic ectoderm and extraembryonic mesoderm also originate from the epiblast.
Chordin is a protein with a prominent role in dorsal–ventral patterning during early embryonic development. In humans it is encoded for by the CHRD gene.
In the field of developmental biology, regional differentiation is the process by which different areas are identified in the development of the early embryo. The process by which the cells become specified differs between organisms.
Convergent extension (CE), sometimes called convergence and extension (C&E), is the process by which the tissue of an embryo is restructured to converge (narrow) along one axis and extend (elongate) along a perpendicular axis by cellular movement.

The development of fishes is unique in some specific aspects compared to the development of other animals.

Homeobox protein goosecoid(GSC) is a homeobox protein that is encoded in humans by the GSC gene. Like other homeobox proteins, goosecoid functions as a transcription factor involved in morphogenesis. In Xenopus, GSC is thought to play a crucial role in the phenomenon of the Spemann-Mangold organizer. Through lineage tracing and timelapse microscopy, the effects of GSC on neighboring cell fates could be observed. In an experiment that injected cells with GSC and observed the effects of uninjected cells, GSC recruited neighboring uninjected cells in the dorsal blastopore lip of the Xenopus gastrula to form a twinned dorsal axis, suggesting that the goosecoid protein plays a role in the regulation and migration of cells during gastrulation.

Hilde Mangold was a German embryologist who was best known for her 1923 dissertation which was the foundation for her mentor, Hans Spemann's, 1935 Nobel Prize in Physiology or Medicine for the discovery of the embryonic organizer, "one of the very few doctoral theses in biology that have directly resulted in the awarding of a Nobel Prize". The general effect she demonstrated is known as embryonic induction, that is, the capacity of some cells to direct the developmental trajectory of other cells. Induction remains a fundamental concept and area of ongoing research in the field.

In avian gastrulation, Koller's sickle is a local thickening of cells at the posterior edge of the upper layer of the area pellucida called the epiblast. Koller's sickle is crucial for avian development, due to its critical role in inducing the differentiation of various avian body parts. Koller's sickle induces primitive streak and Hensen's node, which are major components of avian gastrulation. Avian gastrulation is a process by which developing cells in an avian embryo move relative to one another in order to form the three germ layers.

Johannes Holtfreter was a German-American developmental biologist whose primary focus was the “organizer,” a part of the embryo essential for the development of the proper body plan.
This article is about the role of fibroblast growth factor signaling in mesoderm formation.

A mechanochemical based model for primary neural induction was first proposed in 1985 by Brodland and Gordon. They proposed that there is a mechanically sensitive bistable organelle made of microtubules and microfilaments in the apical ends of cells within cell sheets that are about to differentiate and these cells are under mechanical tension. The microtubules and microfilaments are in mechanical opposition in a proposed embryonic organelle they called the cell state splitter. Depending on where the cell is within a sheet, the tension will be resolved by either the apical end contracting or the apical end expanding. The resolution will begin at one point and spread over the rest of the tissue limited by other mechanical forces at boundaries. An actual physical wave of contraction has been found which traverses the presumptive neural epithelium of the developing salamander, the axolotl. The contraction wave's trajectory was more complex than predicted in the original model however it did originate from the precise location of the Spemann organizer and traversed only the presumptive neural epithelium. Electron microscopy showed intermediate filaments are also present in the cell state splitter. Additional waves of both contraction and expansion were also discovered by time lapse photography of axolotl gastrulation. Among them was a wave of expansion that occurs in ectoderm only in the presumptive epithelium. When the trajectories of the waves were superimposed on the fate map of the axolotl it was shown that there is a unique combination of expansion and contraction waves that correlates with the tissue types determined during gastrulation and that this set of wave trajectories could explain the shape of the fate map.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.
A developmental signaling center is defined as a group of cells that release various morphogens which can determine the fates, or destined cell types, of adjacent cells. This process in turn determines what tissues the adjacent cells will form. Throughout the years, various development signaling centers have been discovered.
References
- 1 2 3 Spemann, Hans (2001). "Induction of embryonic primordia by implantation of organizers from a different species". International Journal of Developmental Biology. 45 (1): 13–38. PMID 11291841.
- 1 2 Vonica, Alin (Dec 1, 2007). "The Xenopus Nieuwkoop Center and Spemann–Mangold Organizer Share Molecular Components and a Requirement for Maternal Wnt Activity". Developmental Biology. 312 (1): 90–102. doi:10.1016/j.ydbio.2007.09.039. PMC 2170525 . PMID 17964564.
- ↑ Niehrs, Christof (June 1, 2004). "Regionally Specific Induction by the Spemann–Mangold Organizer". Nature Reviews Genetics. 5 (6): 425–434. doi:10.1038/nrg1347. PMID 15153995. S2CID 13176134.
- ↑ Pownall, Mary Elizabeth (2010). FGF Signalling in Vertebrate Development (1 ed.). Morgan & Claypool Life Sciences. ISBN 978-1615040636.
- 1 2 3 Sudou, N (May 2012). "Dynamic in Vivo Binding of Transcription Factors to Cis-Regulatory Modules of Cer and Gsc in the Stepwise Formation of the Spemann-Mangold Organizer". Development. 139 (9): 1651–1661. doi:10.1242/dev.068395. PMC 4074222 . PMID 22492356.
- 1 2 Bae, Sangwoo (April 15, 2011). "Siamois and Twin Are Redundant and Essential in Formation of the Spemann Organizer". Developmental Biology. 352 (2): 367–381. doi:10.1016/j.ydbio.2011.01.034. PMC 3065516 . PMID 21295564.
- 1 2 De Robertis, Edward M (April 2006). "Spemann's Organizer and Self-Regulation in Amphibian Embryos". Nature Reviews Molecular Cell Biology. 7 (4): 296–302. doi:10.1038/nrm1855. PMC 2464568 . PMID 16482093.
- 1 2 Cho, Ken W.y. (December 20, 1991). "Molecular Nature of Spemann's Organizer: the Role of the Xenopus Homeobox Gene Goosecoid". Cell. 67 (6): 1111–1120. doi:10.1016/0092-8674(91)90288-a. PMC 3102583 . PMID 1684739.
- 1 2 3 4 Asashima, Makoto (2001). "Spemann's influence on Japanese developmental biology". International Journal of Developmental Biology. 45 (1): 57–65. PMID 11291871.
- 1 2 3 Grunz, Horst (2001). "Developmental biology of amphibians after Hans Spemann in Germany". International Journal of Developmental Biology. 45 (1): 39–50. PMID 11291869.
- 1 2 Mikhailov, Alexander (2001). "Consequences of the Spemann-Mangold organizer concept for embryological research in Russia: Personal impressions". International Journal of Developmental Biology. 45 (1): 83–96. PMID 11291874.