Developmental biology is the study of the process by which animals and plants grow and develop. Developmental biology also encompasses the biology of regeneration, asexual reproduction, metamorphosis, and the growth and differentiation of stem cells in the adult organism.

An embryo is the initial stage of development for a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.

Evolutionary developmental biology is a field of biological research that compares the developmental processes of different organisms to infer how developmental processes evolved.

Gastrulation is the stage in the early embryonic development of most animals, during which the blastula, or in mammals the blastocyst, is reorganized into a two-layered or three-layered embryo known as the gastrula. Before gastrulation, the embryo is a continuous epithelial sheet of cells; by the end of gastrulation, the embryo has begun differentiation to establish distinct cell lineages, set up the basic axes of the body, and internalized one or more cell types including the prospective gut.

Regeneration in biology is the process of renewal, restoration, and tissue growth that makes genomes, cells, organisms, and ecosystems resilient to natural fluctuations or events that cause disturbance or damage. Every species is capable of regeneration, from bacteria to humans. Regeneration can either be complete where the new tissue is the same as the lost tissue, or incomplete after which the necrotic tissue becomes fibrotic.

Somitogenesis is the process by which somites form. Somites are bilaterally paired blocks of paraxial mesoderm that form along the anterior-posterior axis of the developing embryo in segmented animals. In vertebrates, somites give rise to skeletal muscle, cartilage, tendons, endothelium, and dermis.

A morphogen is a substance whose non-uniform distribution governs the pattern of tissue development in the process of morphogenesis or pattern formation, one of the core processes of developmental biology, establishing positions of the various specialized cell types within a tissue. More specifically, a morphogen is a signaling molecule that acts directly on cells to produce specific cellular responses depending on its local concentration.
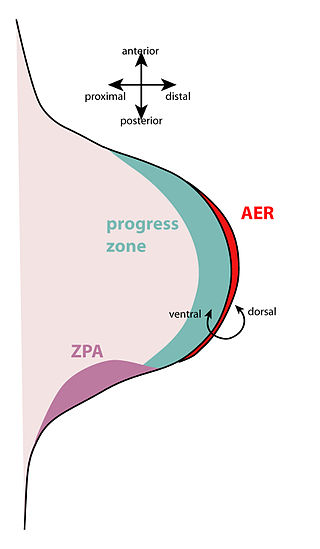
The apical ectodermal ridge (AER) is a structure that forms from the ectodermal cells at the distal end of each limb bud and acts as a major signaling center to ensure proper development of a limb. After the limb bud induces AER formation, the AER and limb mesenchyme—including the zone of polarizing activity (ZPA)—continue to communicate with each other to direct further limb development.
Limb development in vertebrates is an area of active research in both developmental and evolutionary biology, with much of the latter work focused on the transition from fin to limb.
The limb bud is a structure formed early in vertebrate limb development. As a result of interactions between the ectoderm and underlying mesoderm, formation occurs roughly around the fourth week of development. In the development of the human embryo the upper limb bud appears in the third week and the lower limb bud appears four days later.

Paired-like homeodomain transcription factor 2 also known as pituitary homeobox 2 is a protein that in humans is encoded by the PITX2 gene.

Homeobox protein Hox-D13 is a protein that in humans is encoded by the HOXD13 gene. This gene belongs to the homeobox family of genes. The homeobox genes encode a highly conserved family of transcription factors that play an important role in morphogenesis in all multicellular organisms.
Homeotic genes are genes which regulate the development of anatomical structures in various organisms such as echinoderms, insects, mammals, and plants. Homeotic genes often encode transcription factor proteins, and these proteins affect development by regulating downstream gene networks involved in body patterning.

Rosa Susan Penelope Beddington FRS was a British biologist whose career had a major impact on developmental biology.

Elizabeth Jane Robertson is a British developmental biologist based at the Sir William Dunn School of Pathology, University of Oxford. She is Professor of Developmental Biology at Oxford and a Wellcome Trust Principal Research Fellow. She is best known for her pioneering work in developmental genetics, showing that genetic mutations could be introduced into the mouse germ line by using genetically altered embryonic stem cells. This discovery opened up a major field of experimentation for biologists and clinicians.

Hox genes play a massive role in some amphibians and reptiles in their ability to regenerate lost limbs, especially HoxA and HoxD genes.

Homeobox protein CDX-4 is a protein that in humans is encoded by the CDX4 gene. This gene is a member of the caudal-related homeobox transcription factor family that also includes CDX1 and CDX2.
The Spemann-Mangold organizer is a group of cells that are responsible for the induction of the neural tissues during development in amphibian embryos. First described in 1924 by Hans Spemann and Hilde Mangold, the introduction of the organizer provided evidence that the fate of cells can be influenced by factors from other cell populations. This discovery significantly impacted the world of developmental biology and fundamentally changed the understanding of early development.

The dorsal lip of the blastopore is a structure that forms during early embryonic development and is important for its role in organizing the germ layers. The dorsal lip is formed during early gastrulation as folding of tissue along the involuting marginal zone of the blastocoel forms an opening known as the blastopore. It is particularly important for its role in neural induction through the default model, where signaling from the dorsal lip protects a region of the epiblast from becoming epidermis, thus allowing it to develop to its default neural tissue.
A developmental signaling center is defined as a group of cells that release various morphogens which can determine the fates, or destined cell types, of adjacent cells. This process in turn determines what tissues the adjacent cells will form. Throughout the years, various development signaling centers have been discovered.















