
A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.
Poneratoxin is a paralyzing neurotoxic peptide made by the bullet ant Paraponera clavata. It prevents inactivation of voltage gated sodium channels and therefore blocks the synaptic transmission in the central nervous system. Specifically, poneratoxin acts on voltage gated sodium channels in skeletal muscle fibers, causing paralysis, and nociceptive fibers, causing pain. It is rated as a 4 plus on the Schmidt sting pain index, the highest possible rating with that system, and its effects can cause waves of pain up to twelve hours after a single sting. Schmidt describes it as "pure, intense, brilliant pain...like walking over flaming charcoal with a three-inch nail embedded in your heel." It is additionally being studied for its uses in biological insecticides.

Delta atracotoxin is a low-molecular-weight neurotoxic polypeptide found in the venom of the Sydney funnel-web spider.
Taicatoxin (TCX) is a snake toxin that blocks voltage-dependent L-type calcium channels and small conductance Ca2+-activated K+ channels. The name taicatoxin (TAIpan + CAlcium + TOXIN) is derived from its natural source, the taipan snake, the site of its action, calcium channels, and from its function as a toxin. Taicatoxin was isolated from the venom of Australian taipan snake, Oxyuranus scutellatus scutellatus. TCX is a secreted protein, produced in the venom gland of the snake.
The P-type calcium channel is a type of voltage-dependent calcium channel. Similar to many other high-voltage-gated calcium channels, the α1 subunit determines most of the channel's properties. The 'P' signifies cerebellar Purkinje cells, referring to the channel's initial site of discovery. P-type calcium channels play a similar role to the N-type calcium channel in neurotransmitter release at the presynaptic terminal and in neuronal integration in many neuronal types.

Agatoxins are a class of chemically diverse polyamine and peptide toxins which are isolated from the venom of various spiders. Their mechanism of action includes blockade of glutamate-gated ion channels, voltage-gated sodium channels, or voltage-dependent calcium channels. Agatoxin is named after the funnel web spider which produces a venom containing several agatoxins. There are different agatoxins. The ω-agatoxins are approximately 100 amino acids in length and are antagonists of voltage-sensitive calcium channels and also block the release of neurotransmitters. For instance, the ω-agatoxin 1A is a selective blocker and will block L-type calcium channels whereas the ω-agatoxin 4B will inhibit voltage sensitive P-type calcium channels. The μ-agatoxins only act on insect voltage-gated sodium channels.
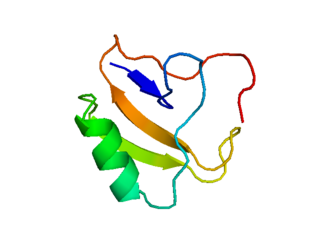
Scorpion toxins are proteins found in the venom of scorpions. Their toxic effect may be mammal- or insect-specific and acts by binding with varying degrees of specificity to members of the Voltage-gated ion channel superfamily; specifically, voltage-gated sodium channels, voltage-gated potassium channels, and Transient Receptor Potential (TRP) channels. The result of this action is to activate or inhibit the action of these channels in the nervous and cardiac organ systems. For instance, α-scorpion toxins MeuNaTxα-12 and MeuNaTxα-13 from Mesobuthus eupeus are neurotoxins that target voltage-gated Na+ channels (Navs), inhibiting fast inactivation. In vivo assays of MeuNaTxα-12 and MeuNaTxα-13 effects on mammalian and insect Navs show differential potency. These recombinants exhibit their preferential affinity for mammalian and insect Na+ channels at the α-like toxins' active site, site 3, in order to inactivate the cell membrane depolarization faster[6]. The varying sensitivity of different Navs to MeuNaTxα-12 and MeuNaTxα-13 may be dependent on the substitution of a conserved Valine residue for a Phenylalanine residue at position 1630 of the LD4:S3-S4 subunit or due to various changes in residues in the LD4:S5-S6 subunit of the Navs. Ultimately, these actions can serve the purpose of warding off predators by causing pain or to subdue predators.
AETX refers to a group of polypeptide neurotoxins isolated from the sea anemone Anemonia erythraea that target ion channels, altering their function. Four subtypes have been identified: AETX I, II, III and K, which vary in their structure and target.
omega-Atracotoxin (ω-atracotoxin) is an insect-specific neurotoxin produced by the Blue Mountains funnel-web spider. Its phylogenetic specificity derives from its ability to antagonise insect, but not vertebrate, voltage-gated calcium channels. Two spatially proximal amino acid residues, Asn(27) and Arg(35), form a contiguous molecular surface that is essential for toxin activity. It has been proposed that this surface of the beta-hairpin is a key site for interaction of the toxin with insect calcium channels.
Jingzhaotoxin proteins are part of a venom secreted by Chilobrachys jingzhao, the Chinese tarantula. and act as neurotoxins. There are several subtypes of jingzhaotoxin, which differ in terms of channel selectivity and modification characteristics. All subspecies act as gating modifiers of sodium channels and/or, to a lesser extent, potassium channels.
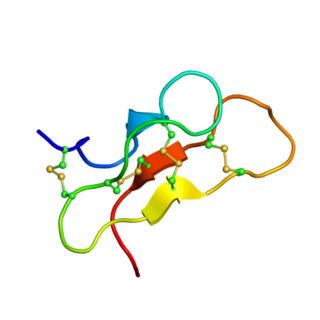
delta-Palutoxins (δ-palutoxins) consist of a homologous group of four insect-specific toxins from the venom of the spider Pireneitega luctuosa. They show a high toxicity against Spodoptera litura larvae by inhibiting sodium channels, leading to strong paralytic activity and eventually to the death of the insect.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.
Hanatoxin is a toxin found in the venom of the Grammostola spatulata tarantula. The toxin is mostly known for inhibiting the activation of voltage-gated potassium channels, most specifically Kv4.2 and Kv2.1, by raising its activation threshold.
Raventoxins are neurotoxins from the venom of the spider Macrothele raveni.
Huwentoxins (HWTX) are a group of neurotoxic peptides found in the venom of the Chinese bird spider Haplopelma schmidti. The species was formerly known as Haplopelma huwenum, Ornithoctonus huwena and Selenocosmia huwena. While structural similarity can be found among several of these toxins, HWTX as a group possess high functional diversity.
Agelenin, also called U1-agatoxin-Aop1a, is an antagonist of the presynaptic P-type calcium channel in insects. This neurotoxic peptide consists of 35 amino acids and can be isolated from the venom of the spider Allagelena opulenta.
APETx1 is a peptide toxin from the venom of the sea anemone Anthopleura elegantissima. The toxin acts as a gating modifier on the human ether-à-go-go-related gene (hERG) channel, a type of voltage-gated potassium channel, and as a blocker of voltage-gated sodium channels, including Nav1.2 and Nav1.8.
Beta-mammal toxin Cn2, also known as Cn2 toxin, is a single chain β-scorpion neurotoxic peptide and the primary toxin in the venom of the Centruroides noxius Hoffmann scorpion. The toxin specifically targets mammalian Nav1.6 voltage-gated sodium channels (VGSC).
Centruroides noxius is a species of scorpion native to Mexico.
Prototoxin-1, or Beta/omega-theraphotoxin-Tp1a, is a 35-amino-acid peptide neurotoxin extracted from the venom of the tarantula Thrixopelma pruriens. Prototoxin-1 belongs to the inhibitory cystine knot (ICK) family of peptide toxins, which have been known to potently inhibit voltage-gated ion channels. Prototoxin-1 selectively blocks low voltage threshold “T-type” calcium channels., voltage gated sodium channels and the nociceptor cation channel TRPA1. Due to its unique ability to bind to TRPA1, Prototoxin-1 has been implicated as a valuable pharmacological reagent with potential applications in clinical contexts with regards to pain and inflammation






