Related Research Articles
In condensed matter physics and materials science, an amorphous solid is a solid that lacks the long-range order that is characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymously with amorphous solid; however, these terms refer specifically to amorphous materials that undergo a glass transition. Examples of amorphous solids include glasses, metallic glasses, and certain types of plastics and polymers.

A crystal or crystalline solid is a solid material whose constituents are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification.

Glass is an amorphous (non-crystalline) solid. Because it is often transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass like "a glass" of water, "glasses", and "magnifying glass", are named after the material.

In metalworking and jewelry making, casting is a process in which a liquid metal is delivered into a mold that contains a negative impression of the intended shape. The metal is poured into the mold through a hollow channel called a sprue. The metal and mold are then cooled, and the metal part is extracted. Casting is most often used for making complex shapes that would be difficult or uneconomical to make by other methods.

Heat treating is a group of industrial, thermal and metalworking processes used to alter the physical, and sometimes chemical, properties of a material. The most common application is metallurgical. Heat treatments are also used in the manufacture of many other materials, such as glass. Heat treatment involves the use of heating or chilling, normally to extreme temperatures, to achieve the desired result such as hardening or softening of a material. Heat treatment techniques include annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing and quenching. Although the term heat treatment applies only to processes where the heating and cooling are done for the specific purpose of altering properties intentionally, heating and cooling often occur incidentally during other manufacturing processes such as hot forming or welding.

Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts-Austen (1843–1902). It exists at room temperature in some stainless steels due to the presence of nickel stabilizing the austenite at lower temperatures.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. As per the established international definition, supercooling means ‘cooling a substance below the normal freezing point without solidification’ While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.

An ingot is a piece of relatively pure material, usually metal, that is cast into a shape suitable for further processing. In steelmaking, it is the first step among semi-finished casting products. Ingots usually require a second procedure of shaping, such as cold/hot working, cutting, or milling to produce a useful final product. Non-metallic and semiconductor materials prepared in bulk form may also be referred to as ingots, particularly when cast by mold based methods. Precious metal ingots can be used as currency, or as a currency reserve, as with gold bars.

An amorphous metal is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity and can show metallic luster.

Liquidmetal and Vitreloy are commercial names of a series of amorphous metal alloys developed by a California Institute of Technology (Caltech) research team and marketed by Liquidmetal Technologies. Liquidmetal alloys combine a number of desirable material features, including high tensile strength, excellent corrosion resistance, very high coefficient of restitution and excellent anti-wearing characteristics, while also being able to be heat-formed in processes similar to thermoplastics. Despite the name, they are not liquid at room temperature.

In materials science and solid mechanics, residual stresses are stresses that remain in a solid material after the original cause of the stresses has been removed. Residual stress may be desirable or undesirable. For example, laser peening imparts deep beneficial compressive residual stresses into metal components such as turbine engine fan blades, and it is used in toughened glass to allow for large, thin, crack- and scratch-resistant glass displays on smartphones. However, unintended residual stress in a designed structure may cause it to fail prematurely.

A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regard to material properties.
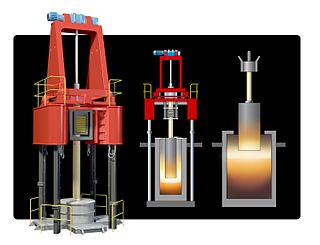
Electroslag remelting (ESR), also known as electro-flux remelting, is a process of remelting and refining steel and other alloys for mission-critical applications in aircraft, thermal power stations, nuclear power plants, military technology and others.
Spray forming, also known as spray casting, spray deposition and in-situ compaction, is a method of casting near net shape metal components with homogeneous microstructures via the deposition of semi-solid sprayed droplets onto a shaped substrate. In spray forming an alloy is melted, normally in an induction furnace, then the molten metal is slowly poured through a conical tundish into a small-bore ceramic nozzle. The molten metal exits the furnace as a thin free-falling stream and is broken up into droplets by an annular array of gas jets, and these droplets then proceed downwards, accelerated by the gas jets to impact onto a substrate. The process is arranged such that the droplets strike the substrate whilst in the semi-solid condition, this provides sufficient liquid fraction to 'stick' the solid fraction together. Deposition continues, gradually building up a spray formed billet of metal on the substrate.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.
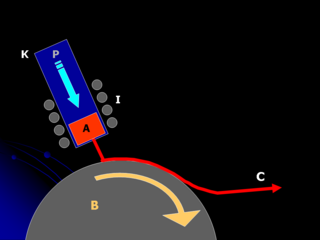
Melt spinning is a metal forming technique that is typically used to form thin ribbons of metal or alloys with a particular atomic structure.
Vacuum arc remelting (VAR) is a secondary melting process for production of metal ingots with elevated chemical and mechanical homogeneity for highly demanding applications. The VAR process has revolutionized the specialty traditional metallurgical techniques industry, and has made possible tightly-controlled materials used in biomedical, aviation and aerospace.

Directional solidification(DS) and progressive solidification are types of solidification within castings. Directional solidification is solidification that occurs from farthest end of the casting and works its way towards the sprue. Progressive solidification, also known as parallel solidification, is solidification that starts at the walls of the casting and progresses perpendicularly from that surface.

The vapor–liquid–solid method (VLS) is a mechanism for the growth of one-dimensional structures, such as nanowires, from chemical vapor deposition. The growth of a crystal through direct adsorption of a gas phase on to a solid surface is generally very slow. The VLS mechanism circumvents this by introducing a catalytic liquid alloy phase which can rapidly adsorb a vapor to supersaturation levels, and from which crystal growth can subsequently occur from nucleated seeds at the liquid–solid interface. The physical characteristics of nanowires grown in this manner depend, in a controllable way, upon the size and physical properties of the liquid alloy.
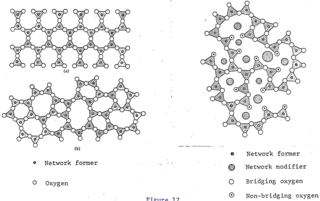
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
References
- ↑ Bennett, T.; Poulikakos D. (1993). "Splat-quench solidification: estimating the maximum spread of a droplet impacting a solid surface". Journal of Materials Science. 28 (4): 2025–2039. Bibcode:1993JMatS..28..963B. doi:10.1007/BF00400880. S2CID 119064426.
- ↑ Davies, H. A.; Hull J. B. (1976). "The formation, structure and crystallization of non-crystalline nickel produced by splat-quenching". Journal of Materials Science. 11 (2): 707–717. Bibcode:1976JMatS..11..215D. doi:10.1007/BF00551430. S2CID 137403190.
- ↑ Bennett, T.; Poulikakos D. (1993). "Splat-quench solidification: estimating the maximum spread of a droplet impacting a solid surface". Journal of Materials Science. 28 (4): 2025–2039. Bibcode:1993JMatS..28..963B. doi:10.1007/BF00400880. S2CID 119064426.
- ↑ Kang, B.; Waldvogel J.; Poulikakos D. (1995). "Remelting phenomena in the process of splat solidification". Journal of Materials Science. 30 (19): 4912–4925. Bibcode:1995JMatS..30.4912K. doi:10.1007/BF01154504. S2CID 136668771.
- ↑ Collings, E. W.; Markworth A. J.; McCoy J. K.; Saunders J. H. (1990). "Splat-quench solidification of freely falling liquid-metal drops by impact on a planar substrate". Journal of Materials Science. 25 (8): 3677–3682. Bibcode:1990JMatS..25.3677C. doi:10.1007/BF00575404. S2CID 135580444.
- ↑ D. V. Louzguine-Luzgin, D. B. Miracle, A. Inoue “Intrinsic and Extrinsic Factors Influencing the Glass-Forming Ability of Alloys” Advanced Engineering Materials, Vol. 10, N: 11, (2008) pp. 1008-1015. DOI: 10.1002/adem.200800134.
- ↑ "Amorphous Solids" . Retrieved 12 November 2012.
- ↑ Rellinghaus, Bernd. "Magnetism in amorphous materials" . Retrieved 13 November 2012.