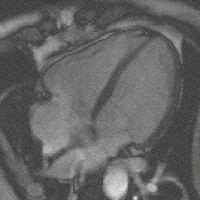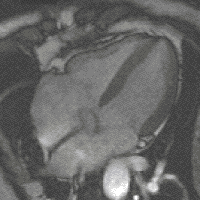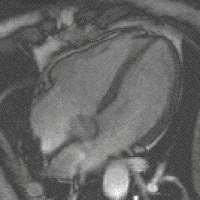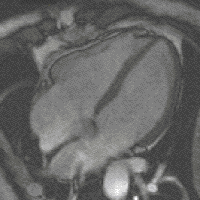
Gradient moments are zero or not
If, within one TR, either one of the gradient moments of magnetic gradients along three logical directions, including slice selection direction (Gss), phase encoding (Gpe) and readout (Gro), is not zero, then spins along such direction obtain different phases, making the signal intensity (SI) of a single voxel the vector sum of magnetizations therein. It causes some inevitable loss of signal. Such situations belong to ordinary SSFP imaging, with its commercial names listed below.
Otherwise, if all gradient moments are zero within one TR, i.e. gradients of opposite polarities cancel out, then there are no additional effects on the phase from gradients; that is to say, SI of each voxels is the contributions of a series of RF pulses and relaxation phenomena. Although the principles underlying echo formation in balanced SSFP have long been known, widespread clinical implementation has been slow due to stringent technical requirements. bSSFP sequences demand a very high level of magnetic field homogeneity and control over gradient switching and shaping. The refocusing mechanism fails if intravoxel dephasing exceeds over ±180º manifest by band-like artifacts. During the last decade modern scanners have overcome these limitations making bSSFP a viable and useful sequence on most mid- and high-field systems. When the echo is recorded close to the middle of the interval (TE ≈ TR/2, as is usually the case), the final term e−TE/T2 depends on T2, not T2*. Thus, bSSFP sequences behave more like spin echo than gradient echo sequences in that they do not have T2*-dependence. Also, since TR is nearly always much, much shorter than T1 or T2, the exponential terms containing TR can be disregarded. [1]
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.