
Steam cleaning involves using steam for cleaning. Its uses include domestic applications in cleaning flooring and household dirt removal, and industrial uses in removing grease and dirt from engines.

Steam cleaning involves using steam for cleaning. Its uses include domestic applications in cleaning flooring and household dirt removal, and industrial uses in removing grease and dirt from engines.
Steam cleaning is not suited for all materials, such as materials which are vapor-sensitive or sensitive for high temperatures. Some examples include silk, some types of plastic, leather, paper, wallpaper and water-based paint. [1]
When used without soap, detergents, or other cleaning products, steam cleaning is an eco-friendly way of cleaning.
Steam cleaning is effective in eliminating 99.9% of bacteria, and is considered a modern way to clean home air-conditioners. [2]
In ovens, steam cleaning is an alternative to catalysis and pyrolysis for making a self-cleaning oven, and uses a lower temperature (approximately 100 Celsius) compared to catalysis (approx. 200 Celsius) and pyrolysis (approx. 500 Celsius). [3]

Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

A kiln is a thermally insulated chamber, a type of oven, that produces temperatures sufficient to complete some process, such as hardening, drying, or chemical changes. Kilns have been used for millennia to turn objects made from clay into pottery, tiles and bricks. Various industries use rotary kilns for pyroprocessing and to transform many other materials.

Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present.

The dew point of a given body of air is the temperature to which it must be cooled to become saturated with water vapor. This temperature depends on the pressure and water content of the air. When the air is cooled below the dew point, its moisture capacity is reduced and airborne water vapor will condense to form liquid water known as dew. When this occurs through the air's contact with a colder surface, dew will form on that surface.

Producer gas is fuel gas that is manufactured by blowing through a coke or coal fire with air and steam simultaneously. It mainly consists of carbon monoxide (CO), hydrogen (H2), as well as substantial amounts of nitrogen (N2). The caloric value of the producer gas is low (mainly because of its high nitrogen content), and the technology is obsolete. Improvements over producer gas, also obsolete, include water gas where the solid fuel is treated intermittently with air and steam and, far more efficiently synthesis gas where the solid fuel is replaced with methane.
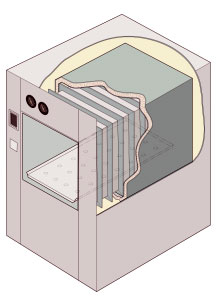
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites.

Pyrolysis is the process of thermal decomposition of materials at elevated temperatures, often in an inert atmosphere without access to oxygen.
In thermodynamics, superheating is the phenomenon in which a liquid is heated to a temperature higher than its boiling point, without boiling. This is a so-called metastable state or metastate, where boiling might occur at any time, induced by external or internal effects. Superheating is achieved by heating a homogeneous substance in a clean container, free of nucleation sites, while taking care not to disturb the liquid.

A clothes dryer is a powered household appliance that is used to remove moisture from a load of clothing, bedding and other textiles, usually after they are washed in the washing machine.

An oven is a tool which is used to expose materials to a hot environment. Ovens contain a hollow chamber and provide a means of heating the chamber in a controlled way. In use since antiquity, they have been used to accomplish a wide variety of tasks requiring controlled heating. Because they are used for a variety of purposes, there are many different types of ovens. These types differ depending on their intended purpose and based upon how they generate heat.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.

A chiller is a machine that removes heat from a liquid coolant via a vapor-compression, adsorption refrigeration, or absorption refrigeration cycles. This liquid can then be circulated through a heat exchanger to cool equipment, or another process stream. As a necessary by-product, refrigeration creates waste heat that must be exhausted to ambience, or for greater efficiency, recovered for heating purposes. Vapor compression chillers may use any of a number of different types of compressors. Most common today are the hermetic scroll, semi-hermetic screw, or centrifugal compressors. The condensing side of the chiller can be either air or water cooled. Even when liquid cooled, the chiller is often cooled by an induced or forced draft cooling tower. Absorption and adsorption chillers require a heat source to function.

Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured.

Hopcalite is the trade name for a number of mixtures that mainly consist of oxides of copper and manganese, which are used as catalysts for the conversion of carbon monoxide to carbon dioxide when exposed to the oxygen in the air at room temperature.
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Most hydrogen is gray hydrogen made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as blue hydrogen.
Laboratory ovens are a common piece of equipment that can be found in electronics, materials processing, forensic, and research laboratories. These ovens generally provide pinpoint temperature control and uniform temperatures throughout the heating process. The following applications are some of the common uses for laboratory ovens: annealing, die-bond curing, drying or dehydrating, Polyimide baking, sterilizing, evaporating. Typical sizes are from one cubic foot to 0.9 cubic metres (32 cu ft). Some ovens can reach temperatures that are higher than 300 degrees Celsius. These temperatures are then applied from all sides of the oven to provide constant heat to sample.
A clean process oven is a type of industrial batch oven that is ideal for high-temperature applications, such as curing polyimide, and annealing thin and film waters. Clean process ovens may be for air atmospheres, or inert atmospheres for oxidation-sensitive materials. Temperatures can be over 525 degrees Celsius.
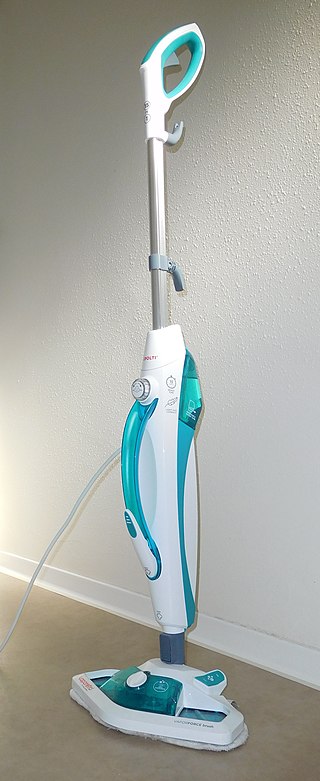
Vapor steam cleaners or steam vapor systems are cleaning appliances or devices that use steam to dry, clean, and sanitize surfaces. The steam is produced in a boiler that heats tap water to high temperatures to produce low-pressure, low moisture water vapor.

Superheated water is liquid water under pressure at temperatures between the usual boiling point, 100 °C (212 °F) and the critical temperature, 374 °C (705 °F). It is also known as "subcritical water" or "pressurized hot water". Superheated water is stable because of overpressure that raises the boiling point, or by heating it in a sealed vessel with a headspace, where the liquid water is in equilibrium with vapour at the saturated vapor pressure. This is distinct from the use of the term superheating to refer to water at atmospheric pressure above its normal boiling point, which has not boiled due to a lack of nucleation sites.

Steam is a substance containing water in the gas phase, often mixed with air and/or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Steam that is saturated or superheated is invisible; however, wet steam, a visible mist or aerosol of water droplets, is often referred to as "steam".