Related Research Articles

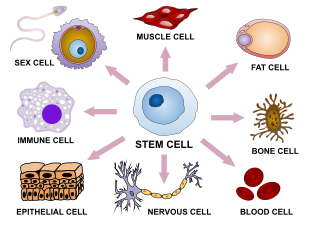
In multicellular organisms, stem cells are undifferentiated or partially differentiated cells that can change into various types of cells and proliferate indefinitely to produce more of the same stem cell. They are the earliest type of cell in a cell lineage. They are found in both embryonic and adult organisms, but they have slightly different properties in each. They are usually distinguished from progenitor cells, which cannot divide indefinitely, and precursor or blast cells, which are usually committed to differentiating into one cell type.
Transdifferentiation, also known as lineage reprogramming, is the process in which one mature somatic cell is transformed into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells. Current uses of transdifferentiation include disease modeling and drug discovery and in the future may include gene therapy and regenerative medicine. The term 'transdifferentiation' was originally coined by Selman and Kafatos in 1974 to describe a change in cell properties as cuticle producing cells became salt-secreting cells in silk moths undergoing metamorphosis.
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. Metabolic composition, however, gets dramatically altered where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.

In genetics and developmental biology, somatic cell nuclear transfer (SCNT) is a laboratory strategy for creating a viable embryo from a body cell and an egg cell. The technique consists of taking a denucleated oocyte and implanting a donor nucleus from a somatic (body) cell. It is used in both therapeutic and reproductive cloning. In 1996, Dolly the sheep became famous for being the first successful case of the reproductive cloning of a mammal. In January 2018, a team of scientists in Shanghai announced the successful cloning of two female crab-eating macaques from foetal nuclei.
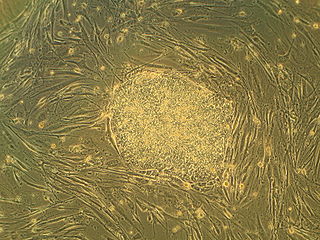
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre-implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist of 50–150 cells. Isolating the inner cell mass (embryoblast) using immunosurgery results in destruction of the blastocyst, a process which raises ethical issues, including whether or not embryos at the pre-implantation stage have the same moral considerations as embryos in the post-implantation stage of development.

Oct-4, also known as POU5F1, is a protein that in humans is encoded by the POU5F1 gene. Oct-4 is a homeodomain transcription factor of the POU family. It is critically involved in the self-renewal of undifferentiated embryonic stem cells. As such, it is frequently used as a marker for undifferentiated cells. Oct-4 expression must be closely regulated; too much or too little will cause differentiation of the cells.
Riken is a national scientific research institute in Japan. Founded in 1917, it now has about 3,000 scientists on seven campuses across Japan, including the main site at Wakō, Saitama Prefecture, on the outskirts of Tokyo. Riken is a Designated National Research and Development Institute, and was formerly an Independent Administrative Institution.
In biology, reprogramming refers to erasure and remodeling of epigenetic marks, such as DNA methylation, during mammalian development or in cell culture. Such control is also often associated with alternative covalent modifications of histones.
Spore-like cells were proposed to be pluripotent cells that lie dormant in animal tissue and become active under stress or injury as adult stem cells, exhibiting behavior characteristic of spores. They were proposed in 2001 by brothers Charles and Martin Vacanti and colleagues. Further work in collaboration with Japanese researchers led to the apparent discovery of STAP cells, in which the pluripotent cells were newly created by stress or injury. This work was published in 2014, but soon found to be due to fraudulent work by Haruko Obokata.

Induced pluripotent stem cells are a type of pluripotent stem cell that can be generated directly from a somatic cell. The iPSC technology was pioneered by Shinya Yamanaka and Kazutoshi Takahashi in Kyoto, Japan, who together showed in 2006 that the introduction of four specific genes, collectively known as Yamanaka factors, encoding transcription factors could convert somatic cells into pluripotent stem cells. Shinya Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon "for the discovery that mature cells can be reprogrammed to become pluripotent."

Shinya Yamanaka is a Japanese stem cell researcher and a Nobel Prize laureate. He is a professor and the director emeritus of Center for iPS Cell Research and Application, Kyoto University; as a senior investigator at the UCSF-affiliated Gladstone Institutes in San Francisco, California; and as a professor of anatomy at University of California, San Francisco (UCSF). Yamanaka is also a past president of the International Society for Stem Cell Research (ISSCR).

Cell potency is a cell's ability to differentiate into other cell types. The more cell types a cell can differentiate into, the greater its potency. Potency is also described as the gene activation potential within a cell, which like a continuum, begins with totipotency to designate a cell with the most differentiation potential, pluripotency, multipotency, oligopotency, and finally unipotency.

Yoshiki Sasai was a Japanese stem cell biologist. He developed methods to guide human embryonic stem cells (hESCs) into forming brain cortex, eyes, and other organs in tissue culture. Sasai worked at the Riken Center for Developmental Biology (CDB) in Kobe, and was Director of the Laboratory for Organogenesis and Neurogenesis. Following his involvement in the 2014 STAP cell controversy, Sasai was found dead at Riken from an apparent suicide.
A Muse cell is an endogenous non-cancerous pluripotent stem cell. They reside in the connective tissue of nearly every organ including the umbilical cord, bone marrow and peripheral blood. They are collectable from commercially obtainable mesenchymal cells such as human fibroblasts, bone marrow-mesenchymal stem cells and adipose-derived stem cells as 1~several percent of the total population. Muse cells are able to generate cells representative of all three germ layers from a single cell both spontaneously and under cytokine induction. Expression of pluripotency genes and triploblastic differentiation are self-renewable over generations. Muse cells do not undergo teratoma formation when transplanted into a host environment in vivo. This can be explained in part by their intrinsically low telomerase activity, eradicating the risk of tumorigenesis through unbridled cell proliferation. They were discovered in 2010 by Mari Dezawa and her research group. Clinical trials for acute myocardial infarction, stroke, epidermolysis bullosa, spinal cord injury, amyotrophic lateral sclerosis, acute respiratory distress syndrome (ARDS) related to novel coronavirus (SARS-CoV-2) infection, are conducted. Physician-led clinical trial for neonatal hypoxic-ischemic encephalopathy was also started. The summary results of a randomized double-blind placebo-controlled clinical trial in patients with stroke was announced.
Haruko Obokata is a former stem-cell biologist and research unit leader at Japan's Laboratory for Cellular Reprogramming, Riken Center for Developmental Biology. She claimed in 2014 to have developed a radical and remarkably easy way to generate stimulus-triggered acquisition of pluripotency (STAP) cells that could be grown into tissue for use anywhere in the body. In response to allegations of irregularities in Obokata's research publications involving STAP cells, Riken launched an investigation that discovered examples of scientific misconduct on the part of Obokata. Attempts to replicate Obokata's STAP cell results failed. The ensuing STAP cell scandal gained worldwide attention.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.
Charles Alfred "Chuck" Vacanti is a researcher in tissue engineering and stem cells and the Vandam/Covino Professor of Anesthesiology, Emeritus, at Harvard Medical School. He is a former head of the Department of Anesthesiology at the University of Massachusetts and Brigham and Women’s Hospital, now retired.
Masayuki Yamato is a professor at Tokyo Women's Medical University. He instructed Haruko Obokata there and wrote a paper on STAP cell with her, Charles Vacanti and Yoshiki Sasai.
Masayo Takahashi is a Japanese medical physician, ophthalmologist and stem cell researcher.

JacobH. Hanna is a Palestinian Arab-Israeli biologist who is working as a professor in the Department of Molecular Genetics at the Weizmann Institute of Science in Rehovot, Israel. An expert in embryonic stem cell research, he is most recognized for developing the first bona fide synthetic embryo models from stem cells in the petri dish in mice and humans.
References
- 1 2 3 4 5 6 7 8 9 10 Cyranoski, David (January 29, 2014). "Acid bath offers easy path to stem cells". Nature News. Nature Publishing Group. Retrieved January 30, 2014.
- ↑ Gallagher, James (January 29, 2014). "Stem cell 'major discovery' claimed". BBC News. Retrieved January 31, 2014.
- 1 2 Kameda, Masaaki; Otake, Tomoko (April 1, 2014). "Obokata falsified data in STAP papers: probe". The Japan Times. Retrieved April 2, 2014.
- 1 2 Elaine Lies (June 4, 2014). "Japan researcher agrees to withdraw disputed stem cell paper". Reuters. Retrieved June 4, 2014.
- ↑ "STAP paper co-author Sasai commits suicide". The Japan Times. Retrieved August 5, 2014.
- 1 2 3 4 5 6 Obokata, Haruko; et al. (January 30, 2014). "Stimulus-triggered fate conversion of somatic cells into pluripotency". Nature. 505 (7485): 641–647. doi:10.1038/nature12968. PMID 24476887. S2CID 4463394. (Retracted, see doi:10.1038/nature13598, PMID 24990753, Retraction Watch)
- ↑ NHS Choices (January 30, 2014). "Breakthrough in stem cell creation using acid bath - What did the research involve?". nhs.co.uk. Retrieved February 6, 2014.
They put them in a weak acid solution (pH 5.7) for 30 minutes at 37°C, and then put them into petri dishes and grew them at normal pH.
- ↑ Thomson, Helen (January 29, 2014). "Stem cell power unleashed after 30 minute dip in acid". New Scientist. Retrieved January 31, 2014.
- 1 2 3 4 5 6 7 Sample, Ian (January 29, 2014). "Simple way to make stem cells in half an hour hailed as major discovery". The Guardian. Retrieved January 31, 2014.
- ↑ Thomson, Helen. "Extraordinary stem cell method tested in human tissue". New Scientist. Retrieved March 7, 2014.
- ↑ Connor, Steve (February 9, 2014). "Exclusive: The miracle cure - scientists turn human skin into stem cells". independent.co.uk. Retrieved February 9, 2014.
- ↑ Grens, Kerry (January 29, 2014). "New Method for Reprogramming Cells". The Scientist.
- ↑ "STAP cell pioneer nearly gave up on her research". The Asahi Shimbun. January 30, 2014. Archived from the original on January 30, 2014.
- ↑ Cyranoski, David (February 17, 2014). "Acid-bath stem-cell study under investigation". Nature . doi:10.1038/nature.2014.14738. S2CID 87071842 . Retrieved February 20, 2014.
- ↑ Haruko Obokata, Yoshiki Sasai and Hitoshi Niwa (March 2014). Essential technical tips for STAP cell conversion culture from somatic cells. Nature Protocols Discussion Forum
- ↑ "Prof. wants STAP findings withdrawn". The Yomiuri Shimbun. March 11, 2014. Archived from the original on March 17, 2014. Retrieved March 17, 2014.
- ↑ Charles A Vacanti (2014)PROTOCOL FOR GENERATING STAP CELLS FROM MATURE SOMATIC CELLS. Center for Tissue Engineering & Regenerative Medicine.
- ↑ Press Release (March 14, 2014). "Interim report on the investigation of the Obokata et al. articles". RIKEN. Retrieved March 17, 2014.
- ↑ Press Release (April 1, 2014). "Report on STAP Cell Research Paper Investigation". RIKEN. Retrieved June 2, 2014.
- ↑ Cyranoski, David (2014). "Research integrity: Cell-induced stress". Nature. 511 (7508): 140–3. Bibcode:2014Natur.511..140C. doi: 10.1038/511140a . PMID 25008506.
- ↑ Otake, Tomoko, "‘STAPgate’ shows Japan must get back to basics in science", Japan Times , April 21, 2014
- ↑ Schreiber, Mark, "Ongoing Obokata story seeks out scandal", Japan Times , July 5, 2014, p. 19
- ↑ Kyodo News, "STAP paper co-author Sasai commits suicide", Japan Times , August 6, 2014, p. 1
- ↑ Konno, Daijiro; Kasukawa, Takeya; Hashimoto, Kosuke; Itoh, Takehiko; Suetsugu, Taeko; Miura, Ikuo; Wakana, Shigeharu; Carninci, Piero; Matsuzaki, Fumio (2015). "STAP cells are derived from ES cells". Nature. 525 (7570): E4 –E5. Bibcode:2015Natur.525E...4K. doi:10.1038/nature15366. ISSN 0028-0836. PMID 26399834. S2CID 4471867.
- ↑ De Los Angeles, Alejandro; Ferrari, Francesco; Fujiwara, Yuko; Mathieu, Ronald; Lee, Soohyun; Lee, Semin; Tu, Ho-Chou; Ross, Samantha; Chou, Stephanie; Nguyen, Minh; Wu, Zhaoting; Theunissen, Thorold W.; Powell, Benjamin E.; Imsoonthornruksa, Sumeth; Chen, Jiekai; Borkent, Marti; Krupalnik, Vladislav; Lujan, Ernesto; Wernig, Marius; Hanna, Jacob H.; Hochedlinger, Konrad; Pei, Duanqing; Jaenisch, Rudolf; Deng, Hongkui; Orkin, Stuart H.; Park, Peter J.; Daley, George Q. (2015). "Failure to replicate the STAP cell phenomenon". Nature. 525 (7570): E6 –E9. Bibcode:2015Natur.525E...6D. doi:10.1038/nature15513. ISSN 0028-0836. PMID 26399835. S2CID 4458211.
- ↑ "STAP revisited". Nature. 525 (7570): 426. 2015. Bibcode:2015Natur.525..426.. doi: 10.1038/525426a . ISSN 0028-0836. PMID 26399791.