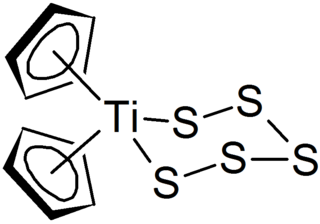A polymer is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.

Bubble gum is a type of chewing gum, designed to be inflated out of the mouth as a bubble.
An elastomer is a polymer with viscoelasticity and with weak intermolecular forces, generally low Young's modulus (E) and high failure strain compared with other materials. The term, a portmanteau of elastic polymer, is often used interchangeably with rubber, although the latter is preferred when referring to vulcanisates. Each of the monomers which link to form the polymer is usually a compound of several elements among carbon, hydrogen, oxygen and silicon. Elastomers are amorphous polymers maintained above their glass transition temperature, so that considerable molecular reconformation is feasible without breaking of covalent bonds. At ambient temperatures, such rubbers are thus relatively compliant and deformable. Their primary uses are for seals, adhesives and molded flexible parts.

Polydimethylsiloxane (PDMS), also known as dimethylpolysiloxane or dimethicone, is a silicone polymer with a wide variety of uses, from cosmetics to industrial lubrication.
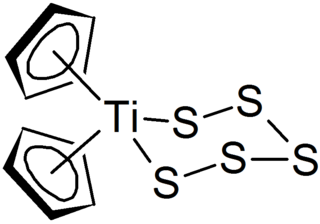
Polysulfides are a class of chemical compounds derived from anionic chains of sulfur atoms. There are two main classes of polysulfides: inorganic and organic. The inorganic polysulfides have the general formula S2−
n. These anions are the conjugate bases of polysulfanes H2Sn. Organic polysulfides generally have the formulae R1SnR2, where R = alkyl or aryl.
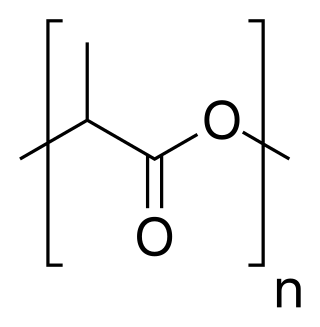
Polylactic acid, also known as poly(lactic acid) or polylactide (PLA), is a thermoplastic polyester with backbone formula (C
3H
4O
2)
n or [–C(CH
3)HC(=O)O–]
n, formally obtained by condensation of lactic acid C(CH
3)(OH)HCOOH with loss of water. It can also be prepared by ring-opening polymerization of lactide [–C(CH
3)HC(=O)O–]
2, the cyclic dimer of the basic repeating unit.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.

Silicone rubber is an elastomer composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from −55 to 300 °C while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants.

The Payne effect is a particular feature of the stress–strain behaviour of rubber, especially rubber compounds containing fillers such as carbon black. It is named after the British rubber scientist A. R. Payne, who made extensive studies of the effect. The effect is sometimes also known as the Fletcher-Gent effect, after the authors of the first study of the phenomenon.

Dielectric elastomers (DEs) are smart material systems that produce large strains and are promising for Soft robotics, Artificial muscle, etc. They belong to the group of electroactive polymers (EAP). DE actuators (DEA) transform electric energy into mechanical work and vice versa. Thus, they can be used as both actuators, sensors, and energy-harvesting devices. They have high elastic energy density and fast response due to being lightweight, highly stretchable, and operating under the electrostatic principle. They have been investigated since the late 1990s. Many prototype applications exist. Every year, conferences are held in the US and Europe.
Shape-memory polymers (SMPs) are polymeric smart materials that have the ability to return from a deformed state to their original (permanent) shape when induced by an external stimulus (trigger), such as temperature change.

Forensic materials engineering, a branch of forensic engineering, focuses on the material evidence from crime or accident scenes, seeking defects in those materials which might explain why an accident occurred, or the source of a specific material to identify a criminal. Many analytical methods used for material identification may be used in investigations, the exact set being determined by the nature of the material in question, be it metal, glass, ceramic, polymer or composite. An important aspect is the analysis of trace evidence such as skid marks on exposed surfaces, where contact between dissimilar materials leaves material traces of one left on the other. Provided the traces can be analysed successfully, then an accident or crime can often be reconstructed. Another aim will be to determine the cause of a broken component using the technique of fractography.

Polymer characterization is the analytical branch of polymer science.

Polyurethane foam is a specialist material used for thermal insulation and other applications. It is a solid polymeric foam based on polyurethane chemistry.
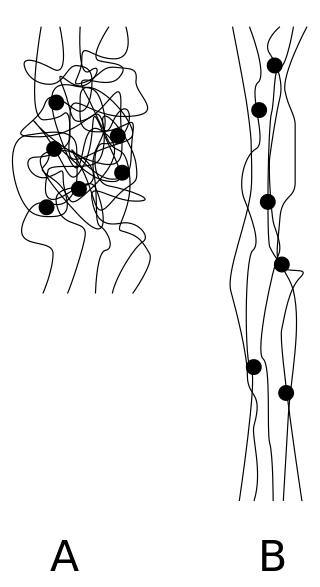
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.
Polymer fracture is the study of the fracture surface of an already failed material to determine the method of crack formation and extension in polymers both fiber reinforced and otherwise. Failure in polymer components can occur at relatively low stress levels, far below the tensile strength because of four major reasons: long term stress or creep rupture, cyclic stresses or fatigue, the presence of structural flaws and stress-cracking agents. Formations of submicroscopic cracks in polymers under load have been studied by x ray scattering techniques and the main regularities of crack formation under different loading conditions have been analyzed. The low strength of polymers compared to theoretically predicted values are mainly due to the many microscopic imperfections found in the material. These defects namely dislocations, crystalline boundaries, amorphous interlayers and block structure can all lead to the non-uniform distribution of mechanical stress.
Liquid crystal elastomers (LCEs) are slightly crosslinked liquid crystalline polymer networks. These materials combine the entropy elasticity of an elastomer with the self-organization of the liquid crystalline phase. In liquid crystalline elastomers, the mesogens can either be part of the polymer chain or are attached via an alkyl spacer.
The polyurethane urea elastomer (PUU), or poly(urethane urea) elastomer, is a flexible polymeric material that is composed of linkages made out of polyurethane and polyurea compounds. Due to its hyperelastic properties, it is capable of bouncing back high-speed ballistic projectiles as if the material had “hardened” upon impact. PUUs were developed by researchers from the U.S. Army Research Laboratory (ARL) and the Army’s Institute for Soldier Nanotechnology at the Massachusetts Institute of Technology (MIT) to potentially replace polyethylene materials in body armor and other protective gear, such as combat helmets, face shields, and ballistic vests.

The thermally induced unidirectional shape-shape-memory effect is an effect classified within the new so-called smart materials. Polymers with thermally induced shape-memory effect are new materials, whose applications are recently being studied in different fields of science, communications and entertainment.