
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products ; this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation, or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the following applications:
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used. It is used to refine crude oil.

Raschig rings are pieces of tube, approximately equal in length and diameter, used in large numbers as a packed bed within columns for distillations and other chemical engineering processes. They are usually ceramic, metal or glass and provide a large surface area within the volume of the column for interaction between liquid and gas vapours. Raschig rings are named after their inventor, German chemist Friedrich Raschig, who patented them in 1914.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.

Vacuum distillation or Distillation under reduced pressure is a type of distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This technique separates compounds based on differences in their boiling points. This technique is used when the boiling point of the desired compound is difficult to achieve or will cause the compound to decompose. Reduced pressures decrease the boiling point of compounds. The reduction in boiling point can be calculated using a temperature-pressure nomograph using the Clausius–Clapeyron relation.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packed bed can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing. Packed beds may also contain catalyst particles or adsorbents such as zeolite pellets, granular activated carbon, etc.

The term structured packing refers to a range of specially designed materials for use in absorption and distillation columns and chemical reactors. Structured packings typically consist of thin corrugated metal plates or gauzes arranged in a way that force fluids to take complicated paths through the column, thereby creating a large surface area for contact between different phases.
The McCabe–Thiele method is a technique that is commonly employed in the field of chemical engineering to model the separation of two substances by a distillation column. It uses the fact that the composition at each theoretical tray is completely determined by the mole fraction of one of the two components. This method is based on the assumptions that the distillation column is isobaric - i.e the presurre remains constant - and that the flow rates of liquid and vapor do not change throughout the column. The assumption of constant molar overflow requires that:
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process.

An evaporator is a type of heat exchanger device that facilitates evaporation by utilizing conductive and convective heat transfer to provide the necessary thermal energy for phase transition from liquid to vapor. Within evaporators, a circulating liquid is exposed to an atmospheric or reduced pressure environment, causing it to boil at a lower temperature compared to normal atmospheric boiling.
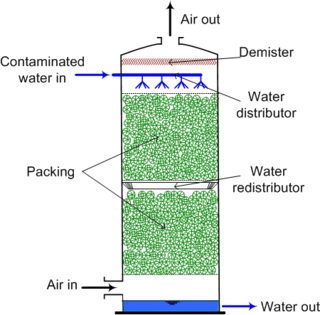
Air stripping is the transferring of volatile components of a liquid into an air stream. It is an environmental engineering technology used for the purification of groundwaters and wastewaters containing volatile compounds.
An oil production plant is a facility which processes production fluids from oil wells in order to separate out key components and prepare them for export. Typical oil well production fluids are a mixture of oil, gas and produced water. An oil production plant is distinct from an oil depot, which does not have processing facilities.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
Glycol dehydration is a liquid desiccant system for the removal of water from natural gas and natural gas liquids (NGL). It is the most common and economical means of water removal from these streams. Glycols typically seen in industry include triethylene glycol (TEG), diethylene glycol (DEG), ethylene glycol (MEG), and tetraethylene glycol (TREG). TEG is the most commonly used glycol in industry.

Electrical resistance heating (ERH) is an intensive in situ environmental remediation method that uses the flow of alternating current electricity to heat soil and groundwater and evaporate contaminants. Electric current is passed through a targeted soil volume between subsurface electrode elements. The resistance to electrical flow that exists in the soil causes the formation of heat; resulting in an increase in temperature until the boiling point of water at depth is reached. After reaching this temperature, further energy input causes a phase change, forming steam and removing volatile contaminants. ERH is typically more cost effective when used for treating contaminant source areas.
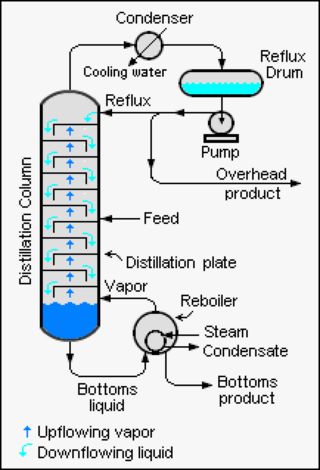
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to reactions over a long period of time.
A plate column is a chemical equipment used to carry out unit operations where it is necessary to transfer mass between a liquid phase and a gas phase. In other words, it is a particular gas-liquid contactor. The peculiarity of this gas-liquid contactor is that the gas comes in contact with liquid through different stages; each stage is delimited by two plates.
Dixon rings are a form of random packing used in chemical processing. They consist of a stainless steel mesh formed into a ring with a central divider, and are intended to be packed randomly into a packed column. Dixon rings provide a large surface area and low pressure drop while maintaining a high mass transfer rate, making them useful for distillations and many other applications.
Random column packing is the practice of packing a distillation column with randomly fitting filtration material in order to optimize surface area over which reactants can interact while minimizing the complexity of construction of such columns. Random column packing is an alternative to structured column packing.













