
A mitochondrion is a double-membrane-bound organelle found in most eukaryotic organisms. Mitochondria generate most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. They were first discovered by Albert von Kölliker in 1880 in the voluntary muscles of insects. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name.

Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.

The electron transport chain (ETC; respiratory chain) is a series of protein complexes that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electron transport chain is built up of peptides, enzymes, and other molecules.

Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from oxygen molecules or nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy because weak high-energy bonds, in particular in molecular oxygen, are replaced by stronger bonds in the products. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it clearly does not resemble one when it occurs in a living cell because of the slow, controlled release of energy from the series of reactions.

A crista is a fold in the inner membrane of a mitochondrion. The name is from the Latin for crest or plume, and it gives the inner membrane its characteristic wrinkled shape, providing a large amount of surface area for chemical reactions to occur on. This aids aerobic cellular respiration, because the mitochondrion requires oxygen. Cristae are studded with proteins, including ATP synthase and a variety of cytochromes.

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An example of this would be the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondria's DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.
Cardiolipin is an important component of the inner mitochondrial membrane, where it constitutes about 20% of the total lipid composition. It can also be found in the membranes of most bacteria. The name "cardiolipin" is derived from the fact that it was first found in animal hearts. It was first isolated from beef heart in the early 1940s. In mammalian cells, but also in plant cells, cardiolipin (CL) is found almost exclusively in the inner mitochondrial membrane, where it is essential for the optimal function of numerous enzymes that are involved in mitochondrial energy metabolism.

In biochemistry and metabolism, beta-oxidation is the catabolic process by which fatty acid molecules are broken down in the cytosol in prokaryotes and in the mitochondria in eukaryotes to generate acetyl-CoA, which enters the citric acid cycle, and NADH and FADH2, which are co-enzymes used in the electron transport chain. It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.

The inner mitochondrial membrane (IMM) is the mitochondrial membrane which separates the mitochondrial matrix from the intermembrane space.
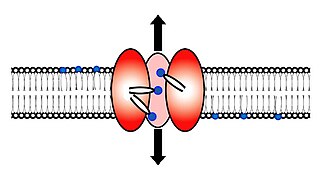
Scramblase is a protein responsible for the translocation of phospholipids between the two monolayers of a lipid bilayer of a cell membrane. In humans, phospholipid scramblases (PLSCRs) constitute a family of five homologous proteins that are named as hPLSCR1–hPLSCR5. Scramblases are not members of the general family of transmembrane lipid transporters known as flippases. Scramblases are distinct from flippases and floppases. Scramblases, flippases, and floppases are three different types of enzymatic groups of phospholipid transportation enzymes. The inner-leaflet, facing the inside of the cell, contains negatively charged amino-phospholipids and phosphatidylethanolamine. The outer-leaflet, facing the outside environment, contains phosphatidylcholine and sphingomyelin. Scramblase is an enzyme, present in the cell membrane, that can transport (scramble) the negatively charged phospholipids from the inner-leaflet to the outer-leaflet, and vice versa.
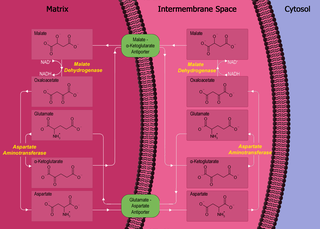
The malate-aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH, the primary reducing equivalent of the electron transport chain. To circumvent this, malate carries the reducing equivalents across the membrane.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines. It can mainly be found in the inner (cytoplasmic) leaflet of the lipid bilayer.

Adenine nucleotide translocator (ANT), also known as the ADP/ATP translocase (ANT), ADP/ATP carrier protein (AAC) or mitochondrial ADP/ATP carrier, exchanges free ATP with free ADP across the inner mitochondrial membrane. ANT is the most abundant protein in the inner mitochondrial membrane and belongs to mitochondrial carrier family.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
The mitochondrial shuttles are systems used to transport reducing agents across the inner mitochondrial membrane. NADH as well as NAD+ cannot cross the membrane, but it can reduce another molecule like FAD and [QH2] that can cross the membrane, so that its electrons can reach the electron transport chain.
Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. The process of mitophagy was first described over a hundred years ago by Margaret Reed Lewis and Warren Harmon Lewis. Ashford and Porter used electron microscopy to observe mitochondrial fragments in liver lysosomes by 1962, and a 1977 report suggested that "mitochondria develop functional alterations which would activate autophagy." The term "mitophagy" was in use by 1998.

The mitochondrial theory of aging has two varieties: free radical and non-free radical. The first is one of the variants of the free radical theory of aging. It was formulated by J. Michel in 1980 and was developed in the works of A. V. Linnan (1989). The second was proposed by A. N. Lobachev in 1978.














