Related Research Articles

Charcot–Marie–Tooth disease (CMT) is a hereditary motor and sensory neuropathy of the peripheral nervous system characterized by progressive loss of muscle tissue and touch sensation across various parts of the body. This disease is the most commonly inherited neurological disorder, affecting about one in 2,500 people. It is named after those who classically described it: the Frenchman Jean-Martin Charcot (1825–1893), his pupil Pierre Marie (1853–1940), and the Briton Howard Henry Tooth (1856–1925).

A molecular lesion or point lesion is damage to the structure of a biological molecule such as DNA, RNA, or protein. This damage may result in the reduction or absence of normal function, and in rare cases the gain of a new function. Lesions in DNA may consist of breaks or other changes in chemical structure of the helix, ultimately preventing transcription. Meanwhile, lesions in proteins consist of both broken bonds and improper folding of the amino acid chain. While many nucleic acid lesions are general across DNA and RNA, some are specific to one, such as thymine dimers being found exclusively in DNA. Several cellular repair mechanisms exist, ranging from global to specific, in order to prevent lasting damage resulting from lesions.

In excitotoxicity, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamate become pathologically high, resulting in excessive stimulation of receptors. For example, when glutamate receptors such as the NMDA receptor or AMPA receptor encounter excessive levels of the excitatory neurotransmitter, glutamate, significant neuronal damage might ensue. Excess glutamate allows high levels of calcium ions (Ca2+) to enter the cell. Ca2+ influx into cells activates a number of enzymes, including phospholipases, endonucleases, and proteases such as calpain. These enzymes go on to damage cell structures such as components of the cytoskeleton, membrane, and DNA. In evolved, complex adaptive systems such as biological life it must be understood that mechanisms are rarely, if ever, simplistically direct. For example, NMDA, in subtoxic amounts, can block glutamate toxicity and thereby induce neuronal survival.

L1, also known as L1CAM, is a transmembrane protein member of the L1 protein family, encoded by the L1CAM gene. This protein, of 200 to 220 kDa, is a neuronal cell adhesion molecule with a strong implication in cell migration, adhesion, neurite outgrowth, myelination and neuronal differentiation. It also plays a key role in treatment-resistant cancers due to its function. It was first identified in 1984 by M. Schachner who found the protein in post-mitotic mice neurons.
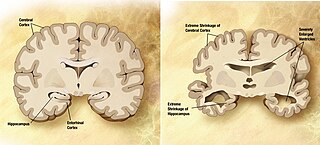
A neurodegenerative disease is caused by the progressive loss of neurons, in the process known as neurodegeneration. Neuronal damage may also ultimately result in their death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.
Neurturin (NRTN) is a protein that is encoded in humans by the NRTN gene. Neurturin belongs to the glial cell line-derived neurotrophic factor (GDNF) family of neurotrophic factors, which regulate the survival and function of neurons. Neurturin’s role as a growth factor places it in the transforming growth factor beta (TGF-beta) subfamily along with its homologs persephin, artemin, and GDNF. It shares a 42% similarity in amino acid sequence with mature GDNF. It is also considered a trophic factor and critical in the development and growth of neurons in the brain. Neurotrophic factors like neurturin have been tested in several clinical trial settings for the potential treatment of neurodegenerative diseases, specifically Parkinson's disease.

Ataxin-1 is a DNA-binding protein which in humans is encoded by the ATXN1 gene.
Sticky mouse is a murine possessing a gene mutation in the enzyme alanyl-tRNA synthetase (AARS). The sticky mouse, with this particular mutation, presents a good model in which to investigate mechanisms of neuronal degeneration. Its most immediately obvious symptom is a sticky secretion on the mouse's fur ; however, it is accompanied by lack of muscle control, ataxia, alopecia, loss of Purkinje cells in the cerebellum, and eventually, death.

Superoxide dismutase [Cu-Zn] also known as superoxide dismutase 1 or hSod1 is an enzyme that in humans is encoded by the SOD1 gene, located on chromosome 21. SOD1 is one of three human superoxide dismutases. It is implicated in apoptosis, familial amyotrophic lateral sclerosis and Parkinson's disease.

Glycine—tRNA ligase also known as glycyl–tRNA synthetase is an enzyme that in humans is encoded by the GARS1 gene.

Neuroferritinopathy is a genetic neurodegenerative disorder characterized by the accumulation of iron in the basal ganglia, cerebellum, and motor cortex of the human brain. Symptoms, which are extrapyramidal in nature, progress slowly and generally do not become apparent until adulthood. These symptoms include chorea, dystonia, and cognitive deficits which worsen with age.

Special AT-rich sequence-binding protein 2 (SATB2) also known as DNA-binding protein SATB2 is a protein that in humans is encoded by the SATB2 gene. SATB2 is a DNA-binding protein that specifically binds nuclear matrix attachment regions and is involved in transcriptional regulation and chromatin remodeling. SATB2 shows a restricted mode of expression and is expressed in certain cell nuclei. The SATB2 protein is mainly expressed in the epithelial cells of the colon and rectum, followed by the nuclei of neurons in the brain.

Paul Reinhard Schimmel is an American biophysical chemist and translational medicine pioneer.

Polyphosphoinositide phosphatase also known as phosphatidylinositol 3,5-bisphosphate 5-phosphatase or SAC domain-containing protein 3 (Sac3) is an enzyme that in humans is encoded by the FIG4 gene. Fig4 is an abbreviation for Factor-Induced Gene.

Alanyl—tRNA synthetase, mitochondrial, also known as alanine—tRNA ligase (AlaRS) or alanyl—tRNA synthetase 2 (AARS2), is an enzyme that in humans is encoded by the AARS2 gene.

Neurodegenerative diseases are a heterogeneous group of complex disorders linked by the degeneration of neurons in either the peripheral nervous system or the central nervous system. Their underlying causes are extremely variable and complicated by various genetic and/or environmental factors. These diseases cause progressive deterioration of the neuron resulting in decreased signal transduction and in some cases even neuronal death. Peripheral nervous system diseases may be further categorized by the type of nerve cell affected by the disorder. Effective treatment of these diseases is often prevented by lack of understanding of the underlying molecular and genetic pathology. Epigenetic therapy is being investigated as a method of correcting the expression levels of misregulated genes in neurodegenerative diseases.
Jeffrey L. Price is an American researcher and author in the fields of circadian rhythms and molecular biology. His chronobiology work with Drosophila melanogaster has led to the discoveries of the circadian genes timeless (tim) and doubletime (dbt), and the doubletime regulators spaghetti (SPAG) and bride of doubletime (BDBT).
There are more than 25 genes known to be associated with amyotrophic lateral sclerosis (ALS) as of June 2018, which collectively account for about 70% of cases of familial ALS (fALS) and 10% of cases of sporadic ALS (sALS). About 5–10% of cases of ALS are directly inherited. Overall, first-degree relatives of an individual with ALS have a 1% risk of developing ALS. ALS has an oligogenic mode of inheritance, meaning that mutations in two or more genes are required to cause disease.
Ted M. Dawson is an American neurologist and neuroscientist. He is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases and director of the Institute for Cell Engineering at Johns Hopkins University School of Medicine. He has joint appointments in the Department of Neurology, Neuroscience and Department of Pharmacology and Molecular Sciences.

Animal models of Parkinson's disease are essential in the research field and widely used to study Parkinson's disease. Parkinson's disease is a neurodegenerative disorder, characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). The loss of the dopamine neurons in the brain, results in motor dysfunction, ultimately causing the four cardinal symptoms of PD: tremor, rigidity, postural instability, and bradykinesia. It is the second most prevalent neurodegenerative disease, following Alzheimer's disease. It is estimated that nearly one million people could be living with PD in the United States.
References
- ↑ "About Susan L. Ackerman, Ph.D." The Jackson Laboratory. Retrieved September 10, 2015.
- 1 2 3 4 5 6 7 8 "Susan L. Ackerman, PhD". HHMI.org. Retrieved 2015-11-19.
- ↑ "National Academy of Sciences Elects Members and Foreign Associates; Historic Number of Women Elected to Its Membership - 2019". National Academy of Sciences. April 30, 2019.
- 1 2 Srivatsa, Swathi; Parthasarathy, Srinivas; Britanova, Olga; Bormuth, Ingo; Donahoo, Amber-Lee; Ackerman, Susan L.; Richards, Linda J.; Tarabykin, Victor (2014-04-17). "Unc5C and DCC act downstream of Ctip2 and Satb2 and contribute to corpus callosum formation". Nature Communications. 5: 3708. Bibcode:2014NatCo...5.3708S. doi:10.1038/ncomms4708. ISSN 2041-1723. PMC 3997811 . PMID 24739528.
- ↑ Klein, Jeffrey A.; Longo-Guess, Chantal M.; Rossmann, Marlies P.; Seburn, Kevin L.; Hurd, Ronald E.; Frankel, Wayne N.; Bronson, Roderick T.; Ackerman, Susan L. (2002-09-26). "The harlequin mouse mutation downregulates apoptosis-inducing factor". Nature. 419 (6905): 367–374. Bibcode:2002Natur.419..367K. doi:10.1038/nature01034. ISSN 0028-0836. PMID 12353028. S2CID 4418288.
- ↑ Jia, Yichang; Mu, John C.; Ackerman, Susan L. (2012-01-20). "Mutation of a U2 snRNA gene causes global disruption of alternative splicing and neurodegeneration". Cell. 148 (1–2): 296–308. doi:10.1016/j.cell.2011.11.057. ISSN 1097-4172. PMC 3488875 . PMID 22265417.
- ↑ Lee, Jeong Woong; Beebe, Kirk; Nangle, Leslie A.; Jang, Jaeseon; Longo-Guess, Chantal M.; Cook, Susan A.; Davisson, Muriel T.; Sundberg, John P.; Schimmel, Paul (2006-09-07). "Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration". Nature. 443 (7107): 50–55. Bibcode:2006Natur.443...50L. doi:10.1038/nature05096. ISSN 1476-4687. PMID 16906134. S2CID 4395135.
- ↑ Ishimura, Ryuta; Nagy, Gabor; Dotu, Ivan; Zhou, Huihao; Yang, Xiang-Lei; Schimmel, Paul; Senju, Satoru; Nishimura, Yasuharu; Chuang, Jeffrey H. (2014-07-25). "RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration". Science. 345 (6195): 455–459. doi:10.1126/science.1249749. ISSN 1095-9203. PMC 4281038 . PMID 25061210.