Related Research Articles

Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).
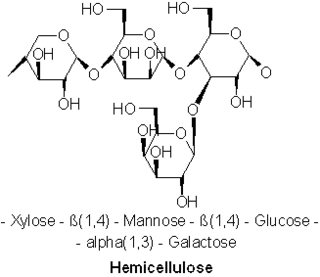
A hemicellulose is one of a number of heteropolymers, such as arabinoxylans, present along with cellulose in almost all terrestrial plant cell walls. Cellulose is crystalline, strong, and resistant to hydrolysis. Hemicelluloses are branched, shorter in length than cellulose, and also show a propensity to crystallize. They can be hydrolyzed by dilute acid or base as well as a myriad of hemicellulase enzymes.

Polysaccharides, or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as hemicellulose and chitin.
In polymer science, the polymer chain or simply backbone of a polymer is the main chain of a polymer. Polymers are often classified according to the elements in the main chains. The character of the backbone, i.e. its flexibility, determines the properties of the polymer. For example, in polysiloxanes (silicone), the backbone chain is very flexible, which results in a very low glass transition temperature of −123 °C. The polymers with rigid backbones are prone to crystallization in thin films and in solution. Crystallization in its turn affects the optical properties of the polymers, its optical band gap and electronic levels.

Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose.

Polythiophenes (PTs) are polymerized thiophenes, a sulfur heterocycle. The parent PT is an insoluble colored solid with the formula (C4H2S)n. The rings are linked through the 2- and 5-positions. Poly(alkylthiophene)s have alkyl substituents at the 3- or 4-position(s). They are also colored solids, but tend to be soluble in organic solvents.

Polydiacetylenes (PDAs) are a family of conducting polymers closely related to polyacetylene. They are created by the 1,4 topochemical polymerization of diacetylenes. They have multiple applications from the development of organic films to immobilization of other molecules.
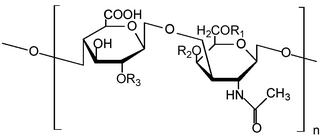
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units. The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers.
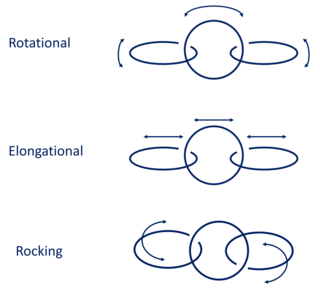
A polycatenane is a chemical substance that, like polymers, is chemically constituted by a large number of units. These units are made up of concatenated rings into a chain-like structure.

In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor and a Michael acceptor to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
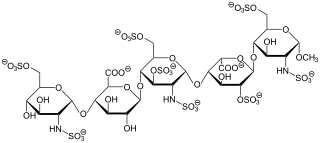
Fondaparinux is an anticoagulant medication chemically related to low molecular weight heparins. It is marketed by Viatris. A generic version developed by Alchemia is marketed within the US by Dr. Reddy's Laboratories.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Glucans are noted in two forms: alpha glucans and beta glucans. Many beta-glucans are medically important. They represent a drug target for antifungal medications of the echinocandin class.
Biodegradable polymers are a special class of polymer that breaks down after its intended purpose by bacterial decomposition process to result in natural byproducts such as gases (CO2, N2), water, biomass, and inorganic salts. These polymers are found both naturally and synthetically made, and largely consist of ester, amide, and ether functional groups. Their properties and breakdown mechanism are determined by their exact structure. These polymers are often synthesized by condensation reactions, ring opening polymerization, and metal catalysts. There are vast examples and applications of biodegradable polymers.

An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst can be heterogeneous such as a platinized electrode. Homogeneous electrocatalysts, which are soluble, assist in transferring electrons between the electrode and reactants, and/or facilitate an intermediate chemical transformation described by an overall half reaction. Major challenges in electrocatalysts focus on fuel cells.
A nanogel is a polymer-based, crosslinked hydrogel particle on the sub-micron scale. These complex networks of polymers present a unique opportunity in the field of drug delivery at the intersection of nanoparticles and hydrogel synthesis. Nanogels can be natural, synthetic, or a combination of the two and have a high degree of tunability in terms of their size, shape, surface functionalization, and degradation mechanisms. Given these inherent characteristics in addition to their biocompatibility and capacity to encapsulate small drugs and molecules, nanogels are a promising strategy to treat disease and dysfunction by serving as delivery vehicles capable of navigating across challenging physiological barriers within the body.
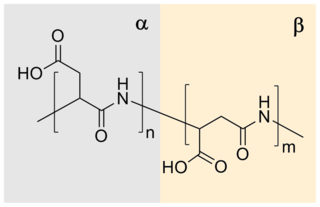
Polyaspartic acid (PASA) is a biodegradable, water-soluble condensation polymer based on the amino acid aspartic acid. It is a biodegradable replacement for water softeners and related applications. PASA can be chemically crosslinked with a wide variety of methods to yield PASA hydrogels. The resulting hydrogels are pH-sensitive such that under acidic conditions, they shrink, while the swelling capacity increases under alkaline conditions.

A sequence-controlled polymer is a macromolecule, in which the sequence of monomers is controlled to some degree. This control can be absolute but not necessarily. In other words, a sequence-controlled polymer can be uniform or non-uniform (Ð>1). For example, an alternating copolymer synthesized by radical polymerization is a sequence-controlled polymer, even if it is also a non-uniform polymer, in which chains have different chain-lengths and slightly different compositions. A biopolymer with a perfectly-defined primary structure is also a sequence-controlled polymer. However, in the case of uniform macromolecules, the term sequence-defined polymer can also be used.
Hydrogels are three-dimensional networks consisting of chemically or physically cross-linked hydrophilic polymers. The insoluble hydrophilic structures absorb polar wound exudates and allow oxygen diffusion at the wound bed to accelerate healing. Hydrogel dressings can be designed to prevent bacterial infection, retain moisture, promote optimum adhesion to tissues, and satisfy the basic requirements of biocompatibility. Hydrogel dressings can also be designed to respond to changes in the microenvironment at the wound bed. Hydrogel dressings should promote an appropriate microenvironment for angiogenesis, recruitment of fibroblasts, and cellular proliferation.
Enzymatic polymerization is a potential area in polymer research, providing a sustainable and adaptable alternative to conventional polymerization processes. Its capacity to manufacture polymers with exact structures in mild circumstances opens up new possibilities for material design and application, helping to progress both research and industry. It is a novel and sustainable method of synthesizing polymers that utilizes the catalytic properties of enzymes to both initiate and regulate the polymerization process. It works under mild circumstances, usually at room temperature and pressure as well as in aqueous environments, in contrast to conventional chemical polymerization techniques that frequently need for harsh conditions and harmful reagents. This approach allows fine control over the structure and functionality of polymers while simultaneously consuming less energy and having a less environmental impact.
References
- 1 2 Vert, Michel; Doi, Yoshiharu; Hellwich, Karl-Heinz; Hess, Michael; Hodge, Philip; Kubisa, Przemyslaw; Rinaudo, Marguerite; Schué, François (11 January 2012). "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)". Pure and Applied Chemistry. 84 (2): 377–410. doi: 10.1351/PAC-REC-10-12-04 . S2CID 98107080.
- 1 2 Kadokawa, Jun-ichi (2011-07-13). "Precision Polysaccharide Synthesis Catalyzed by Enzymes". Chemical Reviews. 111 (7): 4308–4345. doi:10.1021/cr100285v. ISSN 0009-2665. PMID 21319765.
- ↑ Hanson, Sarah; Best, Michael; Bryan, Marian C.; Wong, Chi-Huey (2004-12-01). "Chemoenzymatic synthesis of oligosaccharides and glycoproteins". Trends in Biochemical Sciences. 29 (12): 656–663. doi:10.1016/j.tibs.2004.10.004. ISSN 0968-0004. PMID 15544952.
- ↑ Nilsson, Bradley L.; Soellner, Matthew B.; Raines, Ronald T. (3 May 2005). "Chemical Synthesis of Proteins". Annual Review of Biophysics and Biomolecular Structure. 34 (1): 91–118. doi:10.1146/annurev.biophys.34.040204.144700. ISSN 1056-8700. PMC 2845543 . PMID 15869385.
- ↑ Kent, Stephen B. H. (26 January 2009). "Total chemical synthesis of proteins". Chemical Society Reviews. 38 (2): 338–351. doi:10.1039/B700141J. ISSN 1460-4744. PMID 19169452.
- 1 2 Gross, Richard A.; Ganesh, Manoj; Lu, Wenhua (2010-08-01). "Enzyme-catalysis breathes new life into polyester condensation polymerizations". Trends in Biotechnology. 28 (8): 435–443. doi:10.1016/j.tibtech.2010.05.004. ISSN 0167-7799. PMID 20598389.
- ↑ Natta, G. (1 January 1967). "Crystalline Synthetic High Polymers with a Sterically Regular Structure". Stereoregular Polymers and Stereospecific Polymerizations. Pergamon: 701–707. doi:10.1016/B978-1-4831-9882-8.50055-5. ISBN 9781483198828.
- ↑ Kubicek, Christian P. (2016), "Synthetic Biopolymers", in Glieder, Anton; Kubicek, Christian P.; Mattanovich, Diethard; Wiltschi, Birgit (eds.), Synthetic Biology, Springer International Publishing, pp. 307–335, doi: 10.1007/978-3-319-22708-5_9 , ISBN 9783319227085
- ↑ Rudin, Alfred; Choi, Phillip (2013-01-01), Rudin, Alfred; Choi, Phillip (eds.), "Chapter 11 - Ionic and Coordinated Polymerizations", The Elements of Polymer Science & Engineering (Third Edition), Academic Press, pp. 449–493, doi:10.1016/B978-0-12-382178-2.00011-0, ISBN 9780123821782
- ↑ Song, Jing-She; Huang, Bao-Chen; Yu, Ding-Sheng (2001). "Progress of synthesis and application of trans-1,4-polyisoprene". Journal of Applied Polymer Science. 82 (1): 81–89. doi:10.1002/app.1826. ISSN 1097-4628.
- ↑ Poly(lactic acid) : synthesis, structures, properties, processing and applications. Hoboken, N.J.: Wiley. 2013. ISBN 9781118088135. OCLC 898985627.
- ↑ Reese, Colin B. (2005-10-20). "Oligo- and poly-nucleotides: 50 years of chemical synthesis". Organic & Biomolecular Chemistry. 3 (21): 3851–3868. doi:10.1039/B510458K. ISSN 1477-0539. PMID 16312051.
- ↑ Sohma, Youhei; Pentelute, Brad L.; Whittaker, Jonathan; Hua, Qin-xin; Whittaker, Linda J.; Weiss, Michael A.; Kent, Stephen B. H. (2008). "Comparative Properties of Insulin-like Growth Factor 1 (IGF-1) and [Gly7D-Ala]IGF-1 Prepared by Total Chemical Synthesis". Angewandte Chemie International Edition. 47 (6): 1102–1106. doi:10.1002/anie.200703521. ISSN 1521-3773. PMID 18161716.
- ↑ Sakakibara, Shumpei; Tsuji, Frederick I.; Kimura, Terutoshi; Bódi, József; Nishio, Hideki; Inui, Tatsuya; Nishiuchi, Yuji (1998-11-10). "Chemical synthesis of the precursor molecule of the Aequorea green fluorescent protein, subsequent folding, and development of fluorescence". Proceedings of the National Academy of Sciences. 95 (23): 13549–13554. Bibcode:1998PNAS...9513549N. doi: 10.1073/pnas.95.23.13549 . ISSN 0027-8424. PMC 24856 . PMID 9811837.
- ↑ Kochendoerfer, Gerd G.; Salom, David; Lear, James D.; Wilk-Orescan, Rosemarie; Kent, Stephen B. H.; DeGrado, William F. (1999-09-01). "Total Chemical Synthesis of the Integral Membrane Protein Influenza A Virus M2: Role of Its C-Terminal Domain in Tetramer Assembly". Biochemistry. 38 (37): 11905–11913. doi:10.1021/bi990720m. ISSN 0006-2960. PMID 10508393.
- ↑ Linhardt, Robert J; Liu, Jian (April 2012). "Synthetic heparin". Current Opinion in Pharmacology. 12 (2): 217–219. doi:10.1016/j.coph.2011.12.002. PMC 3496756 . PMID 22325855.
- ↑ Peterson, Sherket; Frick, Amber; Liu, Jian (2009). "Design of biologically active heparan sulfate and heparin using an enzyme-based approach". Natural Product Reports. 26 (5): 610–27. doi:10.1039/B803795G. PMID 19387498.
- ↑ Mende, Marco; Bednarek, Christin; Wawryszyn, Mirella; Sauter, Paul; Biskup, Moritz B.; Schepers, Ute; Bräse, Stefan (13 July 2016). "Chemical Synthesis of Glycosaminoglycans". Chemical Reviews. 116 (14): 8193–8255. doi:10.1021/acs.chemrev.6b00010. PMID 27410264.
- ↑ Pfrengle, Fabian (October 2017). "Synthetic plant glycans". Current Opinion in Chemical Biology. 40: 145–151. doi:10.1016/j.cbpa.2017.09.010. PMID 29024888.
- ↑ Kong, Dehui; Yeung, Wayland; Hili, Ryan (2016-07-11). "Generation of Synthetic Copolymer Libraries by Combinatorial Assembly on Nucleic Acid Templates". ACS Combinatorial Science. 18 (7): 355–370. doi:10.1021/acscombsci.6b00059. ISSN 2156-8952. PMID 27275512.