Avicine, tested and developed by AVI BioPharma, and also known as CTP-37 was trialled as a possible cancer vaccine to treat a number of different cancers. These included colorectal cancer, pancreatic cancer and prostate cancer. The treatment was trialled as and intended to be induced via intramuscular injection into the bloodstream, the location dependent on the treatment area.
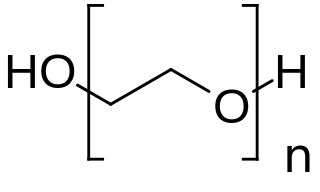
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.

Panobinostat, sold under the brand name Farydak, is a medication used for the treatment of multiple myeloma. It is a hydroxamic acid and acts as a non-selective histone deacetylase inhibitor.
Oncolytics Biotech Inc. is a Canadian company headquartered in Calgary, Alberta, that is developing an intravenously delivered immuno-oncolytic virus called pelareorep for the treatment of solid tumors and hematological malignancies. Pelareorep is a non-pathogenic, proprietary isolate of the unmodified reovirus that: induces selective tumor lysis and promotes an inflamed tumor phenotype through innate and adaptive immune responses.
Pelareorep is a proprietary isolate of the unmodified human reovirus being developed as a systemically administered immuno-oncological viral agent for the treatment of solid tumors and hematological malignancies. Pelareorep is an oncolytic virus, which means that it preferentially lyses cancer cells. Pelareorep also promotes an inflamed tumor phenotype through innate and adaptive immune responses. Preliminary clinical trials indicate that it may have anti-cancer effects across a variety of cancer types when administered alone and in combination with other cancer therapies.
Santaris Pharma A/S was a biopharmaceutical company founded in 2003 in Copenhagen, Denmark. The company also had a branch in San Diego, California that opened in 2009. Created by a merger between Cureon and Pantheco, Santaris developed RNA-targeted medicines using a Locked Nucleic Acid (LNA) Drug Platform and Drug Development Engine.

Perifosine is a former drug candidate that was under development for a variety of cancer indications. It is an alkyl-phospholipid structurally related to miltefosine. Perifosine interrupts the PI3K/AKT/mTOR pathway by acting as an allosteric AKT inhibitor targeting the pleckstrin homology domain of AKT. It was being developed by Keryx Biopharmaceuticals who had licensed it from Æterna Zentaris Inc.

Nanobiotix is a biotechnology company that uses nanomedicine to develop new radiotherapy techniques for cancer patients. The company is headquartered in Paris, with additional corporate offices in New York and Massachusetts.

Idelalisib, sold under the brand name Zydelig, is a medication used to treat certain blood cancers.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.

Sonidegib (INN), sold under the brand name Odomzo, is a medication used to treat cancer.

Venetoclax, sold under the brand names Venclexta and Venclyxto, is a medication used to treat adults with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), or acute myeloid leukemia (AML).
MM-151 is an oligoclonal mixture of fully human monoclonal antibodies, which binds multiple parts of the EGFR molecule It has started clinical trials in patients with RAS wild-type colorectal cancers (CRCs) that were resistant to other anti-EGFR therapies. It is intended to overcome the problem of cancers becoming resistant to monoclonal antibody therapies.

Optimer ligands are short synthetic oligonucleotide molecules composed of DNA or RNA that bind to a specific target molecule. They are engineered to bind their target molecules with affinity typically in the low nanomolar range. Optimers can be used as antibody mimetics in a range of applications, and have been optimized to increase their stability, reduce their molecular weight, and offer increased scalability and consistency in manufacture compared to standard aptamer molecules.

Gedatolisib (PF-05212384) is an experimental drug for treatment of cancer in development by Celcuity, Inc. The mechanism of action is accomplished by binding the different p110 catalytic subunit isoforms of PI3K and the kinase site of mTOR.

Lurbinectedin, sold under the brand name Zepzelca, is a medication used for the treatment of small cell lung cancer.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.
mRNA-5671 also known as V941 is a cancer vaccine candidate developed by Moderna. It is a tetravalent vaccine that targets G12D, G12V, G13D or G12C driver mutations in the KRAS gene. It is currently being evaluated for the treatment of either non-small cell lung cancer, colorectal cancers with microsatellite instability, or pancreatic adenocarcinoma, all with confirmed KRAS driver mutations.









