Related Research Articles

Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
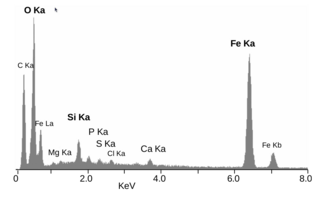
Energy-dispersive X-ray spectroscopy, sometimes called energy dispersive X-ray analysis or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum. The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument.

Hopcalite is the trade name for a number of mixtures that mainly consist of oxides of copper and manganese, which are used as catalysts for the conversion of carbon monoxide to carbon dioxide when exposed to the oxygen in the air at room temperature.

Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of a sample is measured over time as the temperature changes. This measurement provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption; as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.

Raney nickel, also called spongy nickel, is a fine-grained solid composed mostly of nickel derived from a nickel–aluminium alloy. Several grades are known, of which most are gray solids. Some are pyrophoric, but most are used as air-stable slurries. Raney nickel is used as a reagent and as a catalyst in organic chemistry. It was developed in 1926 by American engineer Murray Raney for the hydrogenation of vegetable oils. Raney is a registered trademark of W. R. Grace and Company. Other major producers are Evonik and Johnson Matthey.

Cerium(IV) oxide, also known as ceric oxide, ceric dioxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare-earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial product and an intermediate in the purification of the element from the ores. The distinctive property of this material is its reversible conversion to a non-stoichiometric oxide.
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed. Once the number of moles of each combustion product has been determined the empirical formula or a partial empirical formula of the original compound can be calculated.
The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent and compares it to a reference flow of carrier gas. Since most compounds have a thermal conductivity much less than that of the common carrier gases of helium or hydrogen, when an analyte elutes from the column the effluent thermal conductivity is reduced, and a detectable signal is produced.
PROX is an acronym for PReferential OXidation, that refers to the preferential oxidation of carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and water-gas shift. An ideal PROX catalyst preferentially oxidizes carbon monoxide (CO) using a heterogeneous catalyst placed upon a ceramic support. Catalysts include metals such as platinum, platinum/iron, platinum/ruthenium, gold nanoparticles as well as novel copper oxide/ceramic conglomerate catalysts.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.

In chemistry, a catalyst support is a material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of activated carbon, alumina, and silica.
Operando spectroscopy is an analytical methodology wherein the spectroscopic characterization of materials undergoing reaction is coupled simultaneously with measurement of catalytic activity and selectivity. The primary concern of this methodology is to establish structure-reactivity/selectivity relationships of catalysts and thereby yield information about mechanisms. Other uses include those in engineering improvements to existing catalytic materials and processes and in developing new ones.
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. Methanizers contain a hydrogenation catalyst to achieve this conversion. Nickel is commonly used as the catalyst and there are alternatives available.
The Polyarc reactor is a scientific tool for the measurement of organic molecules. It is paired with a flame ionization detector (FID) in a gas chromatograph (GC) to improve the sensitivity of the FID and give a uniform detector response for all organic molecules (GC-Polyarc/FID).
Praseodymium(III,IV) oxide is the inorganic compound with the formula Pr6O11 that is insoluble in water. It has a cubic fluorite structure. It is the most stable form of praseodymium oxide at ambient temperature and pressure.
References
- ↑ Van 'T Blik, H. F. J.; Niemantsverdriet, J. W. (1984-05-15). "Characterization of bimetallic FeRh/SiO2 catalysts by temperature programmed reduction, oxidation and Mössbauer spectroscopy". Applied Catalysis. 10 (2): 155–162. doi:10.1016/0166-9834(84)80100-1. ISSN 0166-9834. S2CID 58934614.
- ↑ Golunski, S. E. (2008-10-01). "John Ward Jenkins". Platinum Metals Review. 52 (4): 249–250. doi: 10.1595/147106708X366704 .