In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

Gas chromatography–mass spectrometry (GC–MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC–MS include drug detection, fire investigation, environmental analysis, explosives investigation, food and flavor analysis, and identification of unknown samples, including that of material samples obtained from planet Mars during probe missions as early as the 1970s. GC–MS can also be used in airport security to detect substances in luggage or on human beings. Additionally, it can identify trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.

Column chromatography in chemistry is a chromatography method used to isolate a single chemical compound from a mixture. Chromatography is able to separate substances based on differential absorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.

An electron capture detector (ECD) is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by James Lovelock and is used in gas chromatography to detect trace amounts of chemical compounds in a sample.
Combustion analysis is a method used in both organic chemistry and analytical chemistry to determine the elemental composition of a pure organic compound by combusting the sample under conditions where the resulting combustion products can be quantitatively analyzed. Once the number of moles of each combustion product has been determined the empirical formula or a partial empirical formula of the original compound can be calculated.
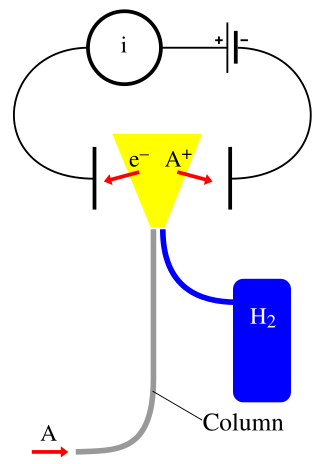
A flame ionization detector (FID) is a scientific instrument that measures analytes in a gas stream. It is frequently used as a detector in gas chromatography. The measurement of ions per unit time makes this a mass sensitive instrument. Standalone FIDs can also be used in applications such as landfill gas monitoring, fugitive emissions monitoring and internal combustion engine emissions measurement in stationary or portable instruments.
Many gas chromatograph detectors are ion detectors with varying methods of ionizing the components eluting from the gas chromatograph's column.
Dissolved gas analysis (DGA) is an examination of electrical transformer oil contaminants. Insulating materials within electrical equipment liberate gases as they slowly break down over time. The composition and distribution of these dissolved gases are indicators of the effects of deterioration, such as pyrolysis or partial discharge, and the rate of gas generation indicates the severity. DGA is beneficial to a preventive maintenance program.
A chromatography detector is a device that detects and quantifies separated compounds as they elute from the chromatographic column. These detectors are integral to various chromatographic techniques, such as gas chromatography, liquid chromatography, and high-performance liquid chromatography, and supercritical fluid chromatography among others. The main function of a chromatography detector is to translate the physical or chemical properties of the analyte molecules into measurable signal, typically electrical signal, that can be displayed as a function of time in a graphical presentation, called a chromatograms. Chromatograms can provide valuable information about the composition and concentration of the components in the sample.
Comprehensive two-dimensional gas chromatography, or GC×GC, is a multidimensional gas chromatography technique that was originally described in 1984 by J. Calvin Giddings and first successfully implemented in 1991 by John Phillips and his student Zaiyou Liu.
A pulsed discharge ionization detector is a detector for gas chromatography that utilizes a stable, low powered, pulsed DC discharge in helium as an ionization source.
Methanizer is an appliance used in gas chromatography (GC), which allows the user to detect very low concentrations of carbon monoxide and carbon dioxide. It consists of a flame ionization detector, preceded by a hydrogenating reactor, which converts CO2 and CO into methane CH4. Methanizers contain a hydrogenation catalyst to achieve this conversion. Nickel is commonly used as the catalyst and there are alternatives available.
Headspace gas chromatography uses headspace gas—from the top or "head" of a sealed container containing a liquid or solid brought to equilibrium—injected directly onto a gas chromatographic column for separation and analysis. In this process, only the most volatile substances make it to the column. The technique is commonly applied to the analysis of polymers, food and beverages, blood alcohol levels, environmental variables, cosmetics, and pharmaceutical ingredients.
A post-column oxidation-reduction reactor is a chemical reactor that performs derivatization to improve the quantitative measurement of organic analytes. It is used in gas chromatography (GC), after the column and before a flame ionization detector (FID), to make the response factor of the detector uniform for all carbon-based species.
Gas chromatography–vacuum ultraviolet spectroscopy (GC-VUV) is a universal detection technique for gas chromatography. VUV detection provides both qualitative and quantitative spectral information for most gas phase compounds.
Gas chromatography-olfactometry (GC-O) is a technique that integrates the separation of volatile compounds using a gas chromatograph with the detection of odour using an olfactometer. It was first invented and applied in 1964 by Fuller and co-workers. While GC separates volatile compounds from an extract, human olfaction detects the odour activity of each eluting compound. In this olfactometric detection, a human assessor may qualitatively determine whether a compound has odour activity or describe the odour perceived, or quantitatively evaluate the intensity of the odour or the duration of the odour activity. The olfactometric detection of compounds allows the assessment of the relationship between a quantified substance and the human perception of its odour, without instrumental detection limits present in other kinds of detectors. Compound identification still requires use of other detectors, such as mass spectrometry, with analytical standards.







