Temperature cycling (or temperature cycle) is the process of cycling through two temperature extremes, typically at relatively high rates of change. It is an environmental stress test used in evaluating product reliability as well as in manufacturing to catch early-term, latent defects by inducing failure through thermal fatigue.

A Carnot heat engine is a theoretical heat engine that operates on the Carnot cycle. The basic model for this engine was developed by Nicolas Léonard Sadi Carnot in 1824. The Carnot engine model was graphically expanded by Benoît Paul Émile Clapeyron in 1834 and mathematically explored by Rudolf Clausius in 1857, work that led to the fundamental thermodynamic concept of entropy. The Carnot engine is the most efficient heat engine which is theoretically possible. The efficiency depends only upon the absolute temperatures of the hot and cold heat reservoirs between which it operates.

A heat engine is a system that converts heat to usable energy, particularly mechanical energy, which can then be used to do mechanical work. While originally conceived in the context of mechanical energy, the concept of the heat engine has been applied to various other kinds of energy, particularly electrical, since at least the late 19th century. The heat engine does this by bringing a working substance from a higher state temperature to a lower state temperature. A heat source generates thermal energy that brings the working substance to the higher temperature state. The working substance generates work in the working body of the engine while transferring heat to the colder sink until it reaches a lower temperature state. During this process some of the thermal energy is converted into work by exploiting the properties of the working substance. The working substance can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings and is not converted to work. Also, some energy is unusable because of friction and drag.

A steam engine is a heat engine that performs mechanical work using steam as its working fluid. The steam engine uses the force produced by steam pressure to push a piston back and forth inside a cylinder. This pushing force can be transformed by a connecting rod and crank into rotational force for work. The term "steam engine" is most commonly applied to reciprocating engines as just described, although some authorities have also referred to the steam turbine and devices such as Hero's aeolipile as "steam engines". The essential feature of steam engines is that they are external combustion engines, where the working fluid is separated from the combustion products. The ideal thermodynamic cycle used to analyze this process is called the Rankine cycle. In general usage, the term steam engine can refer to either complete steam plants, such as railway steam locomotives and portable engines, or may refer to the piston or turbine machinery alone, as in the beam engine and stationary steam engine.

A Stirling engine is a heat engine that is operated by the cyclic expansion and contraction of air or other gas by exposing it to different temperatures, resulting in a net conversion of heat energy to mechanical work.
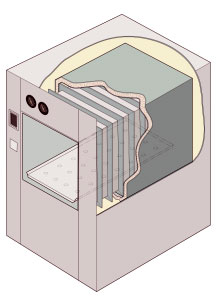
An autoclave is a machine used to carry out industrial and scientific processes requiring elevated temperature and pressure in relation to ambient pressure and/or temperature. Autoclaves are used before surgical procedures to perform sterilization and in the chemical industry to cure coatings and vulcanize rubber and for hydrothermal synthesis. Industrial autoclaves are used in industrial applications, especially in the manufacturing of composites.

The Brayton cycle, also known as the Joule cycle, is a thermodynamic cycle that describes the operation of certain heat engines that have air or some other gas as their working fluid. It is characterized by isentropic compression and expansion, and isobaric heat addition and rejection, though practical engines have adiabatic rather than isentropic steps.

A combined cycle power plant is an assembly of heat engines that work in tandem from the same source of heat, converting it into mechanical energy. On land, when used to make electricity the most common type is called a combined cycle gas turbine (CCGT) plant, which is a kind of gas-fired power plant. The same principle is also used for marine propulsion, where it is called a combined gas and steam (COGAS) plant. Combining two or more thermodynamic cycles improves overall efficiency, which reduces fuel costs.

The Rankine cycle is an idealized thermodynamic cycle describing the process by which certain heat engines, such as steam turbines or reciprocating steam engines, allow mechanical work to be extracted from a fluid as it moves between a heat source and heat sink. The Rankine cycle is named after William John Macquorn Rankine, a Scottish polymath professor at Glasgow University.

A thermal power station, also known as a thermal power plant, is a type of power station in which the heat energy generated from various fuel sources is converted to electrical energy. The heat from the source is converted into mechanical energy using a thermodynamic power cycle. The most common cycle involves a working fluid heated and boiled under high pressure in a pressure vessel to produce high-pressure steam. This high pressure-steam is then directed to a turbine, where it rotates the turbine's blades. The rotating turbine is mechanically connected to an electric generator which converts rotary motion into electricity. Fuels such as natural gas or oil can also be burnt directly in gas turbines, skipping the steam generation step. These plants can be of the open cycle or the more efficient combined cycle type.

Diurnality is a form of plant and animal behavior characterized by activity during daytime, with a period of sleeping or other inactivity at night. The common adjective used for daytime activity is "diurnal". The timing of activity by an animal depends on a variety of environmental factors such as the temperature, the ability to gather food by sight, the risk of predation, and the time of year. Diurnality is a cycle of activity within a 24-hour period; cyclic activities called circadian rhythms are endogenous cycles not dependent on external cues or environmental factors except for a zeitgeber. Animals active during twilight are crepuscular, those active during the night are nocturnal and animals active at sporadic times during both night and day are cathemeral.

A thermodynamic cycle consists of linked sequences of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function.

An absorption refrigerator is a refrigerator that uses a heat source to provide the energy needed to drive the cooling process. Solar energy, burning a fossil fuel, waste heat from factories, and district heating systems are examples of convenient heat sources that can be used. An absorption refrigerator uses two coolants: the first coolant performs evaporative cooling and then is absorbed into the second coolant; heat is needed to reset the two coolants to their initial states. Absorption refrigerators are commonly used in recreational vehicles (RVs), campers, and caravans because the heat required to power them can be provided by a propane fuel burner, by a low-voltage DC electric heater or by a mains-powered electric heater. Absorption refrigerators can also be used to air-condition buildings using the waste heat from a gas turbine or water heater in the building. Using waste heat from a gas turbine makes the turbine very efficient because it first produces electricity, then hot water, and finally, air-conditioning—trigeneration.
Economizers, or economisers (UK), are mechanical devices intended to reduce energy consumption, or to perform useful function such as preheating a fluid. The term economizer is used for other purposes as well. Boiler, power plant, heating, refrigeration, ventilating, and air conditioning (HVAC) may all use economizers. In simple terms, an economizer is a heat exchanger.

Vapour-compression refrigeration or vapor-compression refrigeration system (VCRS), in which the refrigerant undergoes phase changes, is one of the many refrigeration cycles and is the most widely used method for air conditioning of buildings and automobiles. It is also used in domestic and commercial refrigerators, large-scale warehouses for chilled or frozen storage of foods and meats, refrigerated trucks and railroad cars, and a host of other commercial and industrial services. Oil refineries, petrochemical and chemical processing plants, and natural gas processing plants are among the many types of industrial plants that often utilize large vapor-compression refrigeration systems. Cascade refrigeration systems may also be implemented using two compressors.

A Carnot cycle is an ideal thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. By Carnot's theorem, it provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference through the application of work to the system.

A thermal expansion valve or thermostatic expansion valve is a component in vapor-compression refrigeration and air conditioning systems that controls the amount of refrigerant released into the evaporator and is intended to regulate the superheat of the refrigerant that flows out of the evaporator to a steady value. Although often described as a "thermostatic" valve, an expansion valve is not able to regulate the evaporator's temperature to a precise value. The evaporator's temperature will vary only with the evaporating pressure, which will have to be regulated through other means.

Thermodynamic heat pump cycles or refrigeration cycles are the conceptual and mathematical models for heat pump, air conditioning and refrigeration systems. A heat pump is a mechanical system that transmits heat from one location at a certain temperature to another location at a higher temperature. Thus a heat pump may be thought of as a "heater" if the objective is to warm the heat sink, or a "refrigerator" or “cooler” if the objective is to cool the heat source. The operating principles in both cases are the same; energy is used to move heat from a colder place to a warmer place.

In thermal engineering, the organic Rankine cycle (ORC) is a type of thermodynamic cycle. It is a variation of the Rankine cycle named for its use of an organic, high-molecular-mass fluid whose vaporization temperature is lower than that of water. The fluid allows heat recovery from lower-temperature sources such as biomass combustion, industrial waste heat, geothermal heat, solar ponds etc. The low-temperature heat is converted into useful work, that can itself be converted into electricity.
A stellar core is the extremely hot, dense region at the center of a star. For an ordinary main sequence star, the core region is the volume where the temperature and pressure conditions allow for energy production through thermonuclear fusion of hydrogen into helium. This energy in turn counterbalances the mass of the star pressing inward; a process that self-maintains the conditions in thermal and hydrostatic equilibrium. The minimum temperature required for stellar hydrogen fusion exceeds 107 K (10 MK), while the density at the core of the Sun is over 100 g/cm3. The core is surrounded by the stellar envelope, which transports energy from the core to the stellar atmosphere where it is radiated away into space.

An internal combustion engine is a heat engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high-temperature and high-pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons, turbine blades, a rotor, or a nozzle. This force moves the component over a distance. This process transforms chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to.