
The Haber process, also called the Haber–Bosch process, is the main industrial procedure for the production of ammonia. The German chemists Fritz Haber and Carl Bosch developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using an iron metal catalyst under high temperatures and pressures. This reaction is slightly exothermic (i.e. it releases energy), meaning that the reaction is favoured at lower temperatures and higher pressures. It decreases entropy, complicating the process. Hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.

Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.

Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the adsorbate on the surface of the adsorbent. This process differs from absorption, in which a fluid is dissolved by or permeates a liquid or solid. While adsorption does often precede absorption, which involves the transfer of the absorbate into the volume of the absorbent material, alternatively, adsorption is distinctly a surface phenomenon, wherein the adsorbate does not penetrate through the material surface and into the bulk of the adsorbent. The term sorption encompasses both adsorption and absorption, and desorption is the reverse of sorption.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.
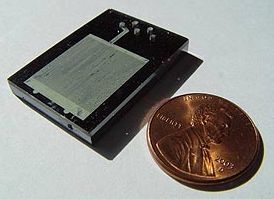
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm; the most typical form of such confinement are microchannels. Microreactors are studied in the field of micro process engineering, together with other devices in which physical processes occur. The microreactor is usually a continuous flow reactor. Microreactors can offer many advantages over conventional scale reactors, including improvements in energy efficiency, reaction speed and yield, safety, reliability, scalability, on-site/on-demand production, and a much finer degree of process control.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
Desorption is the physical process where adsorbed atoms or molecules are released from a surface into the surrounding vacuum or fluid. This occurs when a molecule gains enough energy to overcome the activation barrier and the binding energy that keep it attached to the surface.
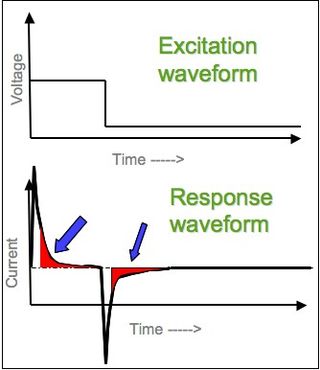
In electrochemistry, chronoamperometry is an analytical technique in which the electric potential of the working electrode is stepped and the resulting current from faradaic processes occurring at the electrode is monitored as a function of time. The functional relationship between current response and time is measured after applying single or double potential step to the working electrode of the electrochemical system. Limited information about the identity of the electrolyzed species can be obtained from the ratio of the peak oxidation current versus the peak reduction current. However, as with all pulsed techniques, chronoamperometry generates high charging currents, which decay exponentially with time as any RC circuit. The Faradaic current - which is due to electron transfer events and is most often the current component of interest - decays as described in the Cottrell equation. In most electrochemical cells, this decay is much slower than the charging decay-cells with no supporting electrolyte are notable exceptions. Most commonly a three-electrode system is used. Since the current is integrated over relatively longer time intervals, chronoamperometry gives a better signal-to-noise ratio in comparison to other amperometric techniques.
Reactions on surfaces are reactions in which at least one of the steps of the reaction mechanism is the adsorption of one or more reactants. The mechanisms for these reactions, and the rate equations are of extreme importance for heterogeneous catalysis. Via scanning tunneling microscopy, it is possible to observe reactions at the solid gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid–gas interface are in some cases related to catalysis.
Sylvia Teresse Ceyer is a professor of chemistry at MIT, holding the John C. Sheehan Chair in Chemistry. Until 2006, she held the chemistry chair of the National Academy of Sciences.

Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters (adparticles) at solid material surfaces. The process can generally be thought of in terms of particles jumping between adjacent adsorption sites on a surface, as in figure 1. Just as in bulk diffusion, this motion is typically a thermally promoted process with rates increasing with increasing temperature. Many systems display diffusion behavior that deviates from the conventional model of nearest-neighbor jumps. Tunneling diffusion is a particularly interesting example of an unconventional mechanism wherein hydrogen has been shown to diffuse on clean metal surfaces via the quantum tunneling effect.
Viktor Vasilyevich Dilman, also spelled Dil'man is a Russian scientist performing research for USPolyResearch. He is best known for his work in chemical engineering and hydrodynamics including the approximate methods for solving nonlinear differential equations of mass, heat, and momentum transfer; mathematical modeling of chemical reactor processes and catalytic distillation; heat, mass, and momentum transfer in turbulent flow; fluid dynamics in granular beds; surface convection, absorption, and molecular convection.

The Sabatier principle is a qualitative concept in chemical heterogeneous catalysis named after the French chemist Paul Sabatier. It states that the interactions between the catalyst and the reactants should be "just right"; that is, neither too strong nor too weak. If the interaction is too weak, the molecule will fail to bind to the catalyst and no reaction will take place. On the other hand, if the interaction is too strong, the product fails to dissociate.
This bibliography of Rutherford Aris contains a comprehensive listing of the scientific publications of Aris, including books, journal articles, and contributions to other published material.
Transition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconducting properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide. Transition metal oxides have a wide variety of surface structures which affect the surface energy of these compounds and influence their chemical properties. The relative acidity and basicity of the atoms present on the surface of metal oxides are also affected by the coordination of the metal cation and oxygen anion, which alter the catalytic properties of these compounds. For this reason, structural defects in transition metal oxides greatly influence their catalytic properties. The acidic and basic sites on the surface of metal oxides are commonly characterized via infrared spectroscopy, calorimetry among other techniques. Transition metal oxides can also undergo photo-assisted adsorption and desorption that alter their electrical conductivity. One of the more researched properties of these compounds is their response to electromagnetic radiation, which makes them useful catalysts for redox reactions, isotope exchange and specialized surfaces.
Carbon nanotube supported catalyst is a novel supported catalyst, using carbon nanotubes as the support instead of the conventional alumina or silicon support. The exceptional physical properties of carbon nanotubes (CNTs) such as large specific surface areas, excellent electron conductivity incorporated with the good chemical inertness, and relatively high oxidation stability makes it a promising support material for heterogeneous catalysis.

Grigoriy Yablonsky is an expert in the area of chemical kinetics and chemical engineering, particularly in catalytic technology of complete and selective oxidation, which is one of the main driving forces of sustainable development.
A post column oxidation-reduction reactor is a chemical reactor that performs derivatization to improve the measurement of organic molecules. It is used in gas chromatography (GC), after the column, and before a flame ionization detector (FID), to make the detector response uniform for all organic molecules.
In chemistry, catalytic resonance theory was developed to describe the kinetics of reaction acceleration using dynamic catalyst surfaces. Catalytic reactions occurring on surfaces that undergo variation in surface binding energy and/or entropy exhibit overall increase in reaction rate when the surface binding energy frequencies are comparable to the natural frequencies of the surface reaction, adsorption, and desorption.

Philippe Sautet is a French chemist. He was elected to the French Academy of sciences on 30 November 2010. He was a research director at the CNRS and works in the chemistry laboratory of the École normale supérieure de Lyon where he devoted a large part of his scientific activity to molecular modelling. Now he is a professor at the University of California - Los Angeles.










