Related Research Articles

β-Galactosidase, is a glycoside hydrolase enzyme that catalyzes hydrolysis of terminal non-reducing β-D-galactose residues in β-D-galactosides.
In genetics, an operon is a functioning unit of DNA containing a cluster of genes under the control of a single promoter. The genes are transcribed together into an mRNA strand and either translated together in the cytoplasm, or undergo splicing to create monocistronic mRNAs that are translated separately, i.e. several strands of mRNA that each encode a single gene product. The result of this is that the genes contained in the operon are either expressed together or not at all. Several genes must be co-transcribed to define an operon.

François Jacob was a French biologist who, together with Jacques Monod, originated the idea that control of enzyme levels in all cells occurs through regulation of transcription. He shared the 1965 Nobel Prize in Medicine with Jacques Monod and André Lwoff.

The lac repressor (LacI) is a DNA-binding protein that inhibits the expression of genes coding for proteins involved in the metabolism of lactose in bacteria. These genes are repressed when lactose is not available to the cell, ensuring that the bacterium only invests energy in the production of machinery necessary for uptake and utilization of lactose when lactose is present. When lactose becomes available, it is firstly converted into allolactose by β-Galactosidase (lacZ) in bacteria. The DNA binding ability of lac repressor bound with allolactose is inhibited due to allosteric regulation, thereby genes coding for proteins involved in lactose uptake and utilization can be expressed.

The lactose operon is an operon required for the transport and metabolism of lactose in E. coli and many other enteric bacteria. Although glucose is the preferred carbon source for most bacteria, the lac operon allows for the effective digestion of lactose when glucose is not available through the activity of beta-galactosidase. Gene regulation of the lac operon was the first genetic regulatory mechanism to be understood clearly, so it has become a foremost example of prokaryotic gene regulation. It is often discussed in introductory molecular and cellular biology classes for this reason. This lactose metabolism system was used by François Jacob and Jacques Monod to determine how a biological cell knows which enzyme to synthesize. Their work on the lac operon won them the Nobel Prize in Physiology in 1965.

Isopropyl β-d-1-thiogalactopyranoside (IPTG) is a molecular biology reagent. This compound is a molecular mimic of allolactose, a lactose metabolite that triggers transcription of the lac operon, and it is therefore used to induce protein expression where the gene is under the control of the lac operator.

Galactoside acetyltransferase is an enzyme that transfers an acetyl group from acetyl-CoA to β-galactosides, glucosides and lactosides. It is coded for by the lacA gene of the lac operon in E. coli.

The trp operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The trp operon was first characterized in Escherichia coli, and it has since been discovered in many other bacteria. The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed.
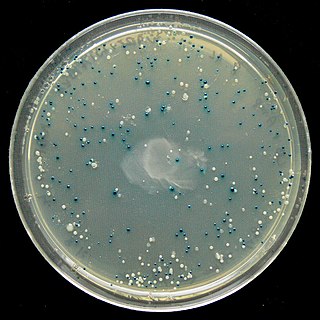
The blue–white screen is a screening technique that allows for the rapid and convenient detection of recombinant bacteria in vector-based molecular cloning experiments. This method of screening is usually performed using a suitable bacterial strain, but other organisms such as yeast may also be used. DNA of transformation is ligated into a vector. The vector is then inserted into a competent host cell viable for transformation, which are then grown in the presence of X-gal. Cells transformed with vectors containing recombinant DNA will produce white colonies; cells transformed with non-recombinant plasmids grow into blue colonies.
The L-arabinose operon, also called the ara or araBAD operon, is an operon required for the breakdown of the five-carbon sugar L-arabinose in Escherichia coli. The L-arabinose operon contains three structural genes: araB, araA, araD, which encode for three metabolic enzymes that are required for the metabolism of L-arabinose. AraB (ribulokinase), AraA, AraD produced by these genes catalyse conversion of L-arabinose to an intermediate of the pentose phosphate pathway, D-xylulose-5-phosphate.
Cold Spring Harbor Laboratory Press was founded in 1933 to aid in Cold Spring Harbor Laboratory's purpose of furthering the advance and spread of scientific knowledge.

DicF RNA is a non-coding RNA that is an antisense inhibitor of cell division gene ftsZ. DicF is bound by the Hfq protein which enhances its interaction with its targets. Pathogenic E. coli strains possess multiple copies of sRNA DicF in their genomes, while non-pathogenic strains do not. DicF and Hfq are both necessary to reduce FtsZ protein levels, leading to cell filamentation under anaerobic conditions.

The enzyme imidazoleglycerol-phosphate dehydratase (EC 4.2.1.19) catalyzes the chemical reaction
The gal operon is a prokaryotic operon, which encodes enzymes necessary for galactose metabolism. Repression of gene expression for this operon works via binding of repressor molecules to two operators. These repressors dimerize, creating a loop in the DNA. The loop as well as hindrance from the external operator prevent RNA polymerase from binding to the promoter, and thus prevent transcription. Additionally, since the metabolism of galactose in the cell is involved in both anabolic and catabolic pathways, a novel regulatory system using two promoters for differential repression has been identified and characterized within the context of the gal operon.
The lacUV5 promoter is a mutated promoter from the Escherichia coli lac operon which is used in molecular biology to drive gene expression on a plasmid. lacUV5 is very similar to the classical lac promoter, containing just 2 base pair mutations in the -10 hexamer region, compared to the lac promoter. LacUV5 is among the most commonly used promoters in molecular biology because it requires no additional activators and it drives high levels of gene expression.

Susan Gottesman is a microbiologist at the National Cancer Institute (NCI), which is part of the National Institutes of Health. Gottesman has been the editor of the Annual Review of Microbiology since 2008.

Daisy Roulland-Dussoix was a Swiss molecular microbiologist. She was one of the discoverers of restriction enzymes during her doctoral studies. There is controversy over whether she should have received the 1978 Nobel prize in Physiology and Medicine, which was awarded to Hamilton O. Smith, Daniel Nathans, and Werner Arber.

Marcos Boris Rotman was a Chilean American immunologist–molecular biologist and professor emeritus of Medical Science at Alpert Medical School of Brown University. He is widely recognized for performing the first single molecule experiments in biology. He died in July 2021 at the age of 96.
Transcription-translation coupling is a mechanism of gene expression regulation in which synthesis of an mRNA (transcription) is affected by its concurrent decoding (translation). In prokaryotes, mRNAs are translated while they are transcribed. This allows communication between RNA polymerase, the multisubunit enzyme that catalyzes transcription, and the ribosome, which catalyzes translation. Coupling involves both direct physical interactions between RNA polymerase and the ribosome, as well as ribosome-induced changes to the structure and accessibility of the intervening mRNA that affect transcription.
Catherine Louise Kearney Squires was a microbiologist known for her work on ribosomal RNA using Escherichia coli as a model organism. She was an elected fellow of the American Academy of Microbiology and the American Association for the Advancement of Science.
References
- ↑ "Terri Grodzicker". Cold Spring Harbor Laboratory. Retrieved July 12, 2020.
- ↑ "Genes & Development -- About the Journal". genesdev.cshlp.org. Retrieved July 12, 2020.
- ↑ "Terri Grodzicker | Book Depository". www.bookdepository.com. Retrieved July 12, 2020.
- ↑ "Wellesley College Alumnae Association". web.wellesley.edu. Retrieved July 12, 2020.
- ↑ "Extensions of Remarks" (PDF). Govinfo.gov. March 19, 1985.
{{cite web}}: CS1 maint: url-status (link) - ↑ Grodzicker, Terri; Zipser, David (December 28, 1968). "A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli". Journal of Molecular Biology. 38 (3): 305–314. doi:10.1016/0022-2836(68)90388-4. ISSN 0022-2836. PMID 4887874.
- ↑ Beckwith, Jon; Grodzicker, Terri; Arditti, Rita (August 14, 1972). "Evidence for two sites in the lac promoter region". Journal of Molecular Biology. 69 (1): 155–160. doi:10.1016/0022-2836(72)90031-9. ISSN 0022-2836. PMID 4341750.
- ↑ Arditti, Rita; Grodzicker, Terri; Beckwith, Jon (May 1, 1973). "Cyclic Adenosine Monophosphate-Independent Mutants of the Lactose Operon of Escherichia coli". Journal of Bacteriology. 114 (2): 652–655. doi:10.1128/JB.114.2.652-655.1973. ISSN 0021-9193. PMC 251822 . PMID 4350344.